Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 3A - Esophagus / Stomach / Practice Management
43 - Cendakimab Efficacy and Safety in Adult and Adolescent Patients With Eosinophilic Esophagitis 48-Week Results From the Randomized, Placebo-Controlled, Phase 3 Study (Late-Breaking Abstract)
Tuesday, October 29, 2024
3:15 PM - 3:25 PM ET
Location: Terrace Ballroom 1

Evan S. Dellon, MD, MPH, FACG
Professor of Medicine and Adjunct Professor of Epidemiology, Center for Esophageal Diseases and Swallowing
University of North Carolina at Chapel Hill
Chapel Hill, NC
Late Breaking Abstract Presenter(s)
Evan S. Dellon, MD, FACG,1 Christina M. Charriez,2 Sandra Zhang,2 Gary Falk,3 Salvatore Oliva,4 Christopher Ma,5 Jesse Siffledeen,6 Shauna Schroeder,7 Hamish Philpott,8 Tim Vanuytsel,9 Christel Contzen,10 Yasuhiko Abe,11 Kexuan Li,2 Carla L. Zema,2 Ashwini Venkatasamy,2 Anusha Yeshokumar,2 Young S. Oh,2 Alain Schoepfer12; 1University of North Carolina School of Medicine at Chapel Hill, Chapel Hill, NC, 2Bristol Myers Squibb, Princeton, NJ, 3University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, 4Azienda Policlinico Umberto I, Rome, Italy, 5University of Calgary, Calgary, AB, Canada, 6University of Alberta, Edmonton, AB, Canada, 7Phoenix Children’s Hospital, Phoenix, AZ, 8Lyell McEwin Hospital, University of Adelaide, Adelaide, South Australia, 9UZ Leuven, Leuven, Flemish Region, Belgium, 10AES – DRS – Synexus, Frankfurt, Germany, 11Yamagata University Hospital, Yamagata, Japan, 12Centre Hospitalier Universitaire Vaudois and University of Lausanne, Lausanne, Switzerland
Introduction: Eosinophilic esophagitis (EoE) is characterized by esophageal inflammation with infiltration of eosinophils (eos) and symptoms of esophageal dysfunction. Interleukin (IL)-13 plays a key role in its pathophysiology. Cendakimab (CEN), a recombinant, humanized, high-affinity, neutralizing monoclonal antibody targeting IL-13, inhibits binding to its receptors IL-13Rα1/Rα2. We report results from a pivotal phase 3 trial (NCT04753697) of CEN in adult and adolescent patients (pts) with EoE after 48 wk of treatment.
Methods: This multicenter, multinational, randomized, double-blind, placebo (PBO)-controlled study enrolled pts aged 12–75 y with EoE (peak esophageal eos count [PEC]) ≥15/high-power field [hpf] at 2 levels of esophagus), >4 dysphagia days (DD) over the 14-day period prior to day 1 evaluated using the modified Daily Symptom Diary (mDSD), and lack of complete response to >8 wk proton pump inhibitor treatment. Pts were randomized 1:1:1 to CEN 360 mg once weekly for 48 wk (QW/QW), CEN 360 mg QW for 24 wk followed by CEN 360 mg every other wk for 24 wk (QW/Q2W), or PBO QW for 48 wk. Coprimary endpoints were mean change from baseline (BL) to wk 24 in DD and proportion of pts with eos histologic response (<6/hpf) at wk 24. Secondary endpoints included mean change from BL to wk 48 in DD, proportion of pts with eos histologic response (PEC <6/hpf and <15/hpf) at wk 48, mean change from BL to wk 48 in EoE Endoscopic Reference Score (EREFS), EoE histology scoring system (EoEHSS) grade/stage, and mDSD composite score; and safety.
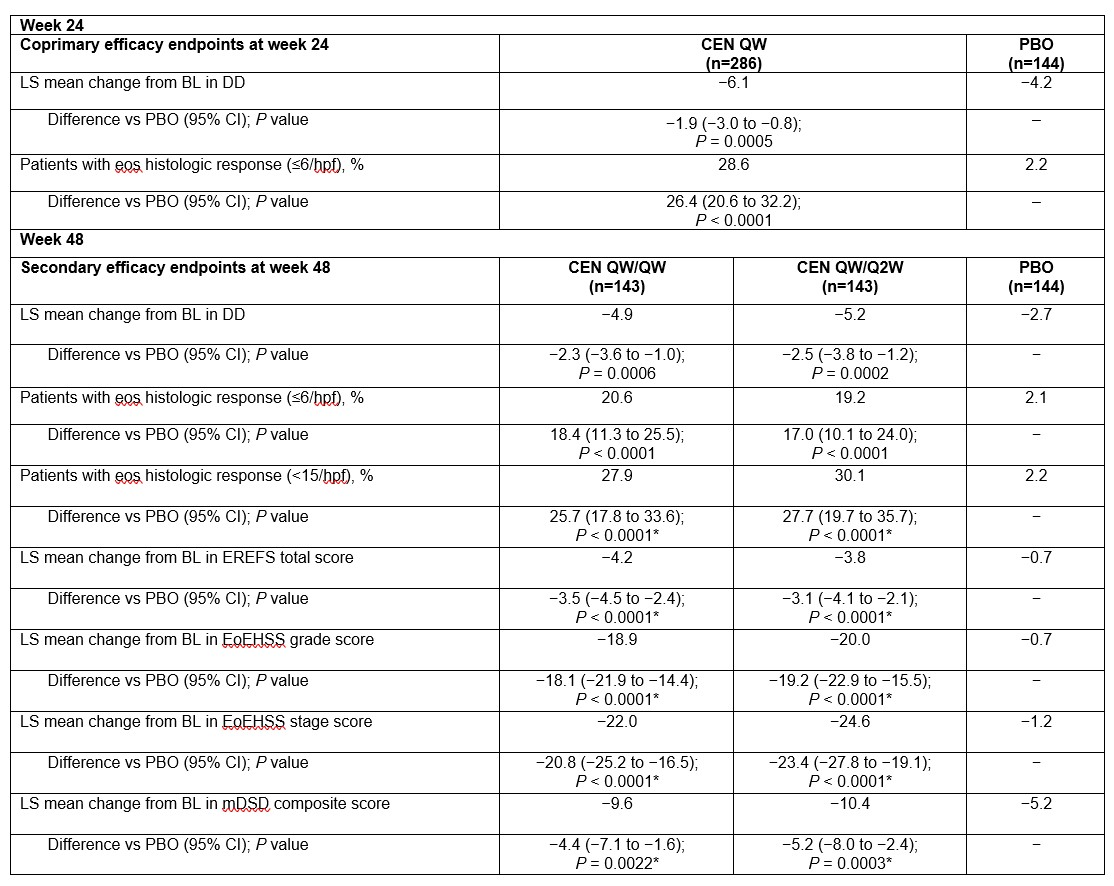
Results: Overall, 430 pts were randomized to CEN QW/QW (n=143), CEN QW/Q2W (n=143), or PBO (n=144); ~66% of pts were steroid inadequate responders/intolerant. In pts in the CEN QW group (n=286), there were significant improvements in coprimary endpoints of change in DD from BL to wk 24 and eos histologic response (<6/hpf) at wk 24 vs PBO (Table). At wk 48, efficacy in secondary endpoints was demonstrated with CEN QW/QW and CEN QW/Q2W vs PBO (Table). Adverse events (AEs) at wk 48 related to study drug occurred in 34.1%, 32.5%, and 21.0% of pts in the CEN QW/QW, CEN QW/Q2W, and PBO arms, with AEs leading to discontinuation in 3.0%, 0.9%, and 1.4% of pts.
Discussion: CEN QW vs PBO demonstrated statistically significant improvements in symptoms and esophageal eos in pts with EoE through 24 wk, which were durable through 48 wk for both QW and Q2W dosing vs PBO. CEN was generally safe and well tolerated through 48 wk.
Disclosures: Evan Dellon - has served as a consultant for Abbott, AbbVie, Adare/Ellodi, Aimmune, Akesobio, Alfasigma, ALK, Allakos, Amgen, Apollo, Aqilion, Arena/Pfizer, Aslan, AstraZeneca, Avir, Biorasi, Bryn, Calypso, Celgene/Receptos/Bristol Myers Squibb, Celldex, Eli Lilly, EsoCap, Eupraxia, Dr Falk Pharma, Ferring, GSK, Gossamer Bio, Holoclara, Invea, Knightpoint, Landos, LucidDx, Morphic, Nexstone Immunology/Uniquity, Nutricia, Parexel/Calyx, Phathom, Regeneron, Revolo, Robarts/Alimentiv, Salix, Sanofi, Shire/Takeda, Target RWE, and Upstream Bio; has received grant/research support from Adare/Ellodi, Allakos, Arena/Pfizer, AstraZeneca, Eupraxia, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/Bristol Myers Squibb, Regeneron, Revolov, and Shire/Takeda; and has received educational grants from Allakos, Aqilion, Holoclara, and Invea. Gary Falk - has served as a consultant for Adare/Ellodi, Allakos, Celgene/Receptos/Bristol Myers Squibb, LucidDx, Nexstone Immunology/Uniquity, Phathom, Regeneron/Sanofi, Shire/Takeda, and Upstream Bio; has received grant/research support from Adare/Ellodi, Allakos, Arena/Pfizer, Celldex, Celgene/Receptos/Bristol Myers Squibb, Regeneron, Sanofi, Revolov, and Shire/Takeda. Alain Schoepfer - has served a consultant for Adare/Ellodi, AbbVie, AstraZeneca, Celgene/Receptos/Bristol Myers Squibb, Dr. Falk Pharma, Gossamer Bio, GSK, Janssen, MSD, Pfizer, Regeneron/Sanofi, Takeda, and Vifor; and has received grant/research support from Adare/Ellodi, Celgene/Receptos/Bristol Myers Squibb, GSK, and Regeneron/Sanofi. Christina Charriez is an employee and shareholder of Bristol Myers Squibb. Sandra Zhang is an employee and shareholder of Bristol Myers Squibb. Kexuan Li is an employee and shareholder of Bristol Myers Squibb. Ashwini Venkatasamy is an employee and shareholder of Bristol Myers Squibb. Anusha Yeshokumar is an employee and shareholder of Bristol Myers Squibb. Young S. Oh is an employee and shareholder of Bristol Myers Squibb. Carla Zema is a contractor to Bristol Myers Squibb. Christopher Ma - has received consulting fees from AbbVie, Alimentiv, Amgen, AVIR Pharma Inc, Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Pendopharm, Pfizer, Prometheus Biosciences Inc., Roche, Sanofi, Takeda, Tillotts Pharma; speaker's fees from AbbVie, Amgen, AVIR Pharma Inc, Alimentiv, Bristol Myers Squibb, Eli Lilly, Ferring, Fresenius Kabi, Janssen, Organon, Pendopharm, Pfizer, Sanofi, Takeda, Tillotts Pharma; royalties from Springer Publishing; research support from AbbVie, Ferring, Pfizer. Hamish Philpott has served as a consultant for Dr Falk Pharma, Arena/Pfizer, GSK and Abbott. Tim Vanuytsel - is a speaker for Dr Falk Pharma and BMS; consultancy for Dr Falk Pharma, BMS; research grant from Dr Falk Pharma. ED has research funding from Adare/Ellodi, Allakos, Arena/Pfizer, AstraZeneca, Celldex, Eupraxia, Ferring, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/BMS, Regeneron, Revolo, Shire/Takeda; is a consultant for Abbvie, Adare/Ellodi, Akesobio, Alfasigma, ALK, Allakos, Amgen, Apollo, Aqilion, Arena/Pfizer, Aslan, AstraZeneca, Avir, Biocryst, Bryn, Calypso, Celgene/Receptos/BMS, Celldex, EsoCap, Eupraxia, Dr. Falk Pharma, Ferring, GI Reviewers, GSK, Holoclara, Invea, Knightpoint, LucidDx, Morphic, Nexstone Immunology/Uniquity, Nutricia, Parexel/Calyx, Phathom, Regeneron, Revolo, Robarts/Alimentiv, Sanofi, Shire/Takeda, Target RWE, Upstream Bio; has an educational grant for Allakos, Aqilion, Holoclara, Invea. Yasuhiko Abe, Jesse Siffledeen, Shauna Schroeder, and Christel Contzen all indicated no relevant financial relationships. Salvatore Oliva - has served a consultant for DrFalk, Medtronic, Regeneron/Sanofi, Celgene/Receptos/Bristol Myers Squibb; and has received grant/research support from Medtronic, Regeneron/Sanofi, Alfa Sigma.
Evan S. Dellon, MD, FACG, Christina M. Charriez, Sandra Zhang, Gary Falk, Salvatore Oliva, Christopher Ma, Jesse Siffledeen, Shauna Schroeder, Hamish Philpott, Tim Vanuytsel, Christel Contzen, Yasuhiko Abe, Kexuan Li, Carla L. Zema, Ashwini Venkatasamy, Anusha Yeshokumar, Young S. Oh, Alain Schoepfer, 43, Cendakimab Efficacy and Safety in Adult and Adolescent Patients With Eosinophilic Esophagitis: 48-Week Results From the Randomized, Placebo-Controlled, Phase 3 Study (late-breaking abstract), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Introduction: Eosinophilic esophagitis (EoE) is characterized by esophageal inflammation with infiltration of eosinophils (eos) and symptoms of esophageal dysfunction. Interleukin (IL)-13 plays a key role in its pathophysiology. Cendakimab (CEN), a recombinant, humanized, high-affinity, neutralizing monoclonal antibody targeting IL-13, inhibits binding to its receptors IL-13Rα1/Rα2. We report results from a pivotal phase 3 trial (NCT04753697) of CEN in adult and adolescent patients (pts) with EoE after 48 wk of treatment.
Methods: This multicenter, multinational, randomized, double-blind, placebo (PBO)-controlled study enrolled pts aged 12–75 y with EoE (peak esophageal eos count [PEC]) ≥15/high-power field [hpf] at 2 levels of esophagus), >4 dysphagia days (DD) over the 14-day period prior to day 1 evaluated using the modified Daily Symptom Diary (mDSD), and lack of complete response to >8 wk proton pump inhibitor treatment. Pts were randomized 1:1:1 to CEN 360 mg once weekly for 48 wk (QW/QW), CEN 360 mg QW for 24 wk followed by CEN 360 mg every other wk for 24 wk (QW/Q2W), or PBO QW for 48 wk. Coprimary endpoints were mean change from baseline (BL) to wk 24 in DD and proportion of pts with eos histologic response (<6/hpf) at wk 24. Secondary endpoints included mean change from BL to wk 48 in DD, proportion of pts with eos histologic response (PEC <6/hpf and <15/hpf) at wk 48, mean change from BL to wk 48 in EoE Endoscopic Reference Score (EREFS), EoE histology scoring system (EoEHSS) grade/stage, and mDSD composite score; and safety.
Results: Overall, 430 pts were randomized to CEN QW/QW (n=143), CEN QW/Q2W (n=143), or PBO (n=144); ~66% of pts were steroid inadequate responders/intolerant. In pts in the CEN QW group (n=286), there were significant improvements in coprimary endpoints of change in DD from BL to wk 24 and eos histologic response (<6/hpf) at wk 24 vs PBO (Table). At wk 48, efficacy in secondary endpoints was demonstrated with CEN QW/QW and CEN QW/Q2W vs PBO (Table). Adverse events (AEs) at wk 48 related to study drug occurred in 34.1%, 32.5%, and 21.0% of pts in the CEN QW/QW, CEN QW/Q2W, and PBO arms, with AEs leading to discontinuation in 3.0%, 0.9%, and 1.4% of pts.
Discussion: CEN QW vs PBO demonstrated statistically significant improvements in symptoms and esophageal eos in pts with EoE through 24 wk, which were durable through 48 wk for both QW and Q2W dosing vs PBO. CEN was generally safe and well tolerated through 48 wk.

Table: Results at wks 24 and 48
Disclosures: Evan Dellon - has served as a consultant for Abbott, AbbVie, Adare/Ellodi, Aimmune, Akesobio, Alfasigma, ALK, Allakos, Amgen, Apollo, Aqilion, Arena/Pfizer, Aslan, AstraZeneca, Avir, Biorasi, Bryn, Calypso, Celgene/Receptos/Bristol Myers Squibb, Celldex, Eli Lilly, EsoCap, Eupraxia, Dr Falk Pharma, Ferring, GSK, Gossamer Bio, Holoclara, Invea, Knightpoint, Landos, LucidDx, Morphic, Nexstone Immunology/Uniquity, Nutricia, Parexel/Calyx, Phathom, Regeneron, Revolo, Robarts/Alimentiv, Salix, Sanofi, Shire/Takeda, Target RWE, and Upstream Bio; has received grant/research support from Adare/Ellodi, Allakos, Arena/Pfizer, AstraZeneca, Eupraxia, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/Bristol Myers Squibb, Regeneron, Revolov, and Shire/Takeda; and has received educational grants from Allakos, Aqilion, Holoclara, and Invea. Gary Falk - has served as a consultant for Adare/Ellodi, Allakos, Celgene/Receptos/Bristol Myers Squibb, LucidDx, Nexstone Immunology/Uniquity, Phathom, Regeneron/Sanofi, Shire/Takeda, and Upstream Bio; has received grant/research support from Adare/Ellodi, Allakos, Arena/Pfizer, Celldex, Celgene/Receptos/Bristol Myers Squibb, Regeneron, Sanofi, Revolov, and Shire/Takeda. Alain Schoepfer - has served a consultant for Adare/Ellodi, AbbVie, AstraZeneca, Celgene/Receptos/Bristol Myers Squibb, Dr. Falk Pharma, Gossamer Bio, GSK, Janssen, MSD, Pfizer, Regeneron/Sanofi, Takeda, and Vifor; and has received grant/research support from Adare/Ellodi, Celgene/Receptos/Bristol Myers Squibb, GSK, and Regeneron/Sanofi. Christina Charriez is an employee and shareholder of Bristol Myers Squibb. Sandra Zhang is an employee and shareholder of Bristol Myers Squibb. Kexuan Li is an employee and shareholder of Bristol Myers Squibb. Ashwini Venkatasamy is an employee and shareholder of Bristol Myers Squibb. Anusha Yeshokumar is an employee and shareholder of Bristol Myers Squibb. Young S. Oh is an employee and shareholder of Bristol Myers Squibb. Carla Zema is a contractor to Bristol Myers Squibb. Christopher Ma - has received consulting fees from AbbVie, Alimentiv, Amgen, AVIR Pharma Inc, Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Pendopharm, Pfizer, Prometheus Biosciences Inc., Roche, Sanofi, Takeda, Tillotts Pharma; speaker's fees from AbbVie, Amgen, AVIR Pharma Inc, Alimentiv, Bristol Myers Squibb, Eli Lilly, Ferring, Fresenius Kabi, Janssen, Organon, Pendopharm, Pfizer, Sanofi, Takeda, Tillotts Pharma; royalties from Springer Publishing; research support from AbbVie, Ferring, Pfizer. Hamish Philpott has served as a consultant for Dr Falk Pharma, Arena/Pfizer, GSK and Abbott. Tim Vanuytsel - is a speaker for Dr Falk Pharma and BMS; consultancy for Dr Falk Pharma, BMS; research grant from Dr Falk Pharma. ED has research funding from Adare/Ellodi, Allakos, Arena/Pfizer, AstraZeneca, Celldex, Eupraxia, Ferring, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/BMS, Regeneron, Revolo, Shire/Takeda; is a consultant for Abbvie, Adare/Ellodi, Akesobio, Alfasigma, ALK, Allakos, Amgen, Apollo, Aqilion, Arena/Pfizer, Aslan, AstraZeneca, Avir, Biocryst, Bryn, Calypso, Celgene/Receptos/BMS, Celldex, EsoCap, Eupraxia, Dr. Falk Pharma, Ferring, GI Reviewers, GSK, Holoclara, Invea, Knightpoint, LucidDx, Morphic, Nexstone Immunology/Uniquity, Nutricia, Parexel/Calyx, Phathom, Regeneron, Revolo, Robarts/Alimentiv, Sanofi, Shire/Takeda, Target RWE, Upstream Bio; has an educational grant for Allakos, Aqilion, Holoclara, Invea. Yasuhiko Abe, Jesse Siffledeen, Shauna Schroeder, and Christel Contzen all indicated no relevant financial relationships. Salvatore Oliva - has served a consultant for DrFalk, Medtronic, Regeneron/Sanofi, Celgene/Receptos/Bristol Myers Squibb; and has received grant/research support from Medtronic, Regeneron/Sanofi, Alfa Sigma.
Evan S. Dellon, MD, FACG, Christina M. Charriez, Sandra Zhang, Gary Falk, Salvatore Oliva, Christopher Ma, Jesse Siffledeen, Shauna Schroeder, Hamish Philpott, Tim Vanuytsel, Christel Contzen, Yasuhiko Abe, Kexuan Li, Carla L. Zema, Ashwini Venkatasamy, Anusha Yeshokumar, Young S. Oh, Alain Schoepfer, 43, Cendakimab Efficacy and Safety in Adult and Adolescent Patients With Eosinophilic Esophagitis: 48-Week Results From the Randomized, Placebo-Controlled, Phase 3 Study (late-breaking abstract), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

