Oral Paper Presentation
Annual Scientific Meeting
Session: Presidential Plenary Session 2
7 - Comparative Effectiveness of Biofeedback and Dextranomer/Hyaluronate Injection for Treatment of Fecal Incontinence: A Multicenter Randomized Controlled Trial
Monday, October 28, 2024
9:42 AM - 9:54 AM ET
Location: Terrace Ballroom
- AB
Adil Bharucha, MBBS, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Award: ACG Outstanding Research Award in the Functional Bowel Disease Category
Adil Bharucha, MBBS, MD1, Satish S. C.. Rao, MD, PhD2, William Whitehead, PhD3, Isuzu Meyer, MD, MSPH4, William D. Chey, MD, FACG5, Jan Busby-Whitehead, MD6, Marie Gantz, PhD7, Heidi Chua, MD1, Stacy Menees, MD8, William Perry, MBChB, MPH1, Holly E.. Richter, PhD, MD9, Amaanti Sridhar, MSc7, Jennifer Wu, MD3, Lawrence Szarka, MD1, Frank Hamilton, MD10
1Mayo Clinic, Rochester, MN; 2Medical College of Georgia, Augusta University, Augusta, GA; 3University of North Carolina at Chapel Hill, Chapel Hill, NC; 4University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 5University of Michigan, Ann Arbor, MI; 6UNC School of Medicine, Chapel Hill, NC; 7RTI International, Research Triangle Park, NC; 8Michigan Medicine, Ann Arbor, MI; 9University of Alabama at Birmingham, Birmingham, AL; 10National Institutes of Health, Bethesda, MD
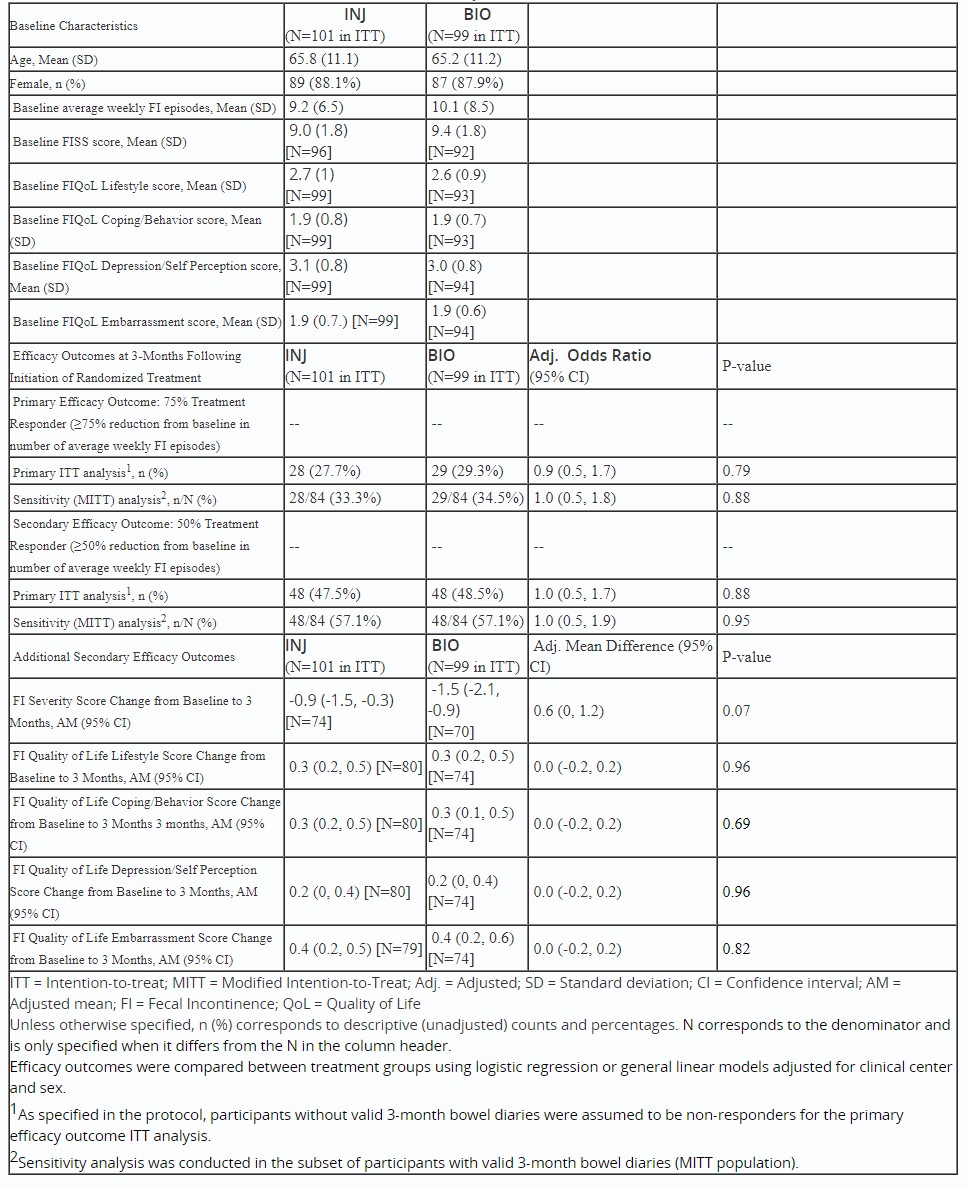
Introduction: Fecal incontinence (FI), affects 10% of community dwelling adults and markedly impairs quality of life. Among patients who fail medical and behavioral therapy, biofeedback (BIO) therapy and anal injection with dextranomer hyaluronate bulking agents (INJ), are recommended evidence-based treatments but have not been compared in a large multicenter randomized clinical trial.
Methods: Ambulatory patients aged ≥18 yrs with FI for ≥6 months, and ≥2 average weekly episodes of FI /week and fulfilled criteria for treatment with BIO/INJ were enrolled at 5 US centers (PMID: 34139357). Patients first received 4 weeks of enhanced medical management (EMM), which included dietary modifications and medications that were tailored to the underlying bowel disturbance(s), and pelvic floor exercises. Non-responders to EMM (i.e., < 75% reduction in average weekly FI episodes vs baseline) were randomized (1:1) to BIO (5-6 sessions) or INJ. The primary efficacy outcome was ≥75% reduction in average weekly FI episodes at 3 months vs baseline (intention to treat, ITT); primary safety outcome was proportion of patients with adverse events (AEs) of pelvic pain of grade II or higher severity, based on common terminology criteria for AEs, treatment site infection, or serious AEs (SAE) requiring hospitalization. Group outcomes were compared using adjusted logistic and general linear models, and for AEs, Fisher’s exact tests. Additional follow up to 24 months is in progress.
Results: 275 participants underwent EMM, 200 were randomized and included in ITT (101 INJ, 99 BIO). 183 initiated treatment (90 INJ, 93 BIO). There was no difference in 75% responder status, 28/101 (27.7%) vs 29/99 (29.3%), respectively, adjusted odds ratio, (95% confidence interval) 0.9 (0.5, 1.7), FI severity score, or FI quality of life scores (Table 1). Eleven AEs relevant to the primary safety outcome were reported in 7 participants (5 INJ, 2 BIO, p-value 0.27). Between randomization and 3-months, 5 participants had SAEs (3 INJ, 2 BIO), none related to the interventions.
Discussion: Biofeedback and dextranomer hyaluronate injection were not significantly different with respect to improvement in FI episodes and symptoms at 3 months in this first, large, multicenter clinical trial of patients with moderately severe FI who failed to respond to EMM. Both treatments should be considered in FI patients who have not adequately responded to conservative measures.
Disclosures:
Adil Bharucha, MBBS, MD1, Satish S. C.. Rao, MD, PhD2, William Whitehead, PhD3, Isuzu Meyer, MD, MSPH4, William D. Chey, MD, FACG5, Jan Busby-Whitehead, MD6, Marie Gantz, PhD7, Heidi Chua, MD1, Stacy Menees, MD8, William Perry, MBChB, MPH1, Holly E.. Richter, PhD, MD9, Amaanti Sridhar, MSc7, Jennifer Wu, MD3, Lawrence Szarka, MD1, Frank Hamilton, MD10, 7, Comparative Effectiveness of Biofeedback and Dextranomer/Hyaluronate Injection for Treatment of Fecal Incontinence: A Multicenter Randomized Controlled Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Adil Bharucha, MBBS, MD1, Satish S. C.. Rao, MD, PhD2, William Whitehead, PhD3, Isuzu Meyer, MD, MSPH4, William D. Chey, MD, FACG5, Jan Busby-Whitehead, MD6, Marie Gantz, PhD7, Heidi Chua, MD1, Stacy Menees, MD8, William Perry, MBChB, MPH1, Holly E.. Richter, PhD, MD9, Amaanti Sridhar, MSc7, Jennifer Wu, MD3, Lawrence Szarka, MD1, Frank Hamilton, MD10
1Mayo Clinic, Rochester, MN; 2Medical College of Georgia, Augusta University, Augusta, GA; 3University of North Carolina at Chapel Hill, Chapel Hill, NC; 4University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 5University of Michigan, Ann Arbor, MI; 6UNC School of Medicine, Chapel Hill, NC; 7RTI International, Research Triangle Park, NC; 8Michigan Medicine, Ann Arbor, MI; 9University of Alabama at Birmingham, Birmingham, AL; 10National Institutes of Health, Bethesda, MD
Introduction: Fecal incontinence (FI), affects 10% of community dwelling adults and markedly impairs quality of life. Among patients who fail medical and behavioral therapy, biofeedback (BIO) therapy and anal injection with dextranomer hyaluronate bulking agents (INJ), are recommended evidence-based treatments but have not been compared in a large multicenter randomized clinical trial.
Methods: Ambulatory patients aged ≥18 yrs with FI for ≥6 months, and ≥2 average weekly episodes of FI /week and fulfilled criteria for treatment with BIO/INJ were enrolled at 5 US centers (PMID: 34139357). Patients first received 4 weeks of enhanced medical management (EMM), which included dietary modifications and medications that were tailored to the underlying bowel disturbance(s), and pelvic floor exercises. Non-responders to EMM (i.e., < 75% reduction in average weekly FI episodes vs baseline) were randomized (1:1) to BIO (5-6 sessions) or INJ. The primary efficacy outcome was ≥75% reduction in average weekly FI episodes at 3 months vs baseline (intention to treat, ITT); primary safety outcome was proportion of patients with adverse events (AEs) of pelvic pain of grade II or higher severity, based on common terminology criteria for AEs, treatment site infection, or serious AEs (SAE) requiring hospitalization. Group outcomes were compared using adjusted logistic and general linear models, and for AEs, Fisher’s exact tests. Additional follow up to 24 months is in progress.
Results: 275 participants underwent EMM, 200 were randomized and included in ITT (101 INJ, 99 BIO). 183 initiated treatment (90 INJ, 93 BIO). There was no difference in 75% responder status, 28/101 (27.7%) vs 29/99 (29.3%), respectively, adjusted odds ratio, (95% confidence interval) 0.9 (0.5, 1.7), FI severity score, or FI quality of life scores (Table 1). Eleven AEs relevant to the primary safety outcome were reported in 7 participants (5 INJ, 2 BIO, p-value 0.27). Between randomization and 3-months, 5 participants had SAEs (3 INJ, 2 BIO), none related to the interventions.
Discussion: Biofeedback and dextranomer hyaluronate injection were not significantly different with respect to improvement in FI episodes and symptoms at 3 months in this first, large, multicenter clinical trial of patients with moderately severe FI who failed to respond to EMM. Both treatments should be considered in FI patients who have not adequately responded to conservative measures.

Disclosures:
Adil Bharucha: Medspira – Intellectual Property/Patents, Royalties. Minnesota Medical Technologies – Grant/Research Support, Intellectual Property/Patents, Royalties.
Satish Rao: Ironwood Pharmaceuticals – Advisory Committee/Board Member, Grant/Research Support. Pallette life sciences – Advisor or Review Panel Member.
William Whitehead indicated no relevant financial relationships.
Isuzu Meyer: Juniper Biomedical – Advisory Committee/Board Member.
William Chey: AbbVie – Consultant. Ardelyx – Consultant. Atmo BioSciences – Consultant. Comvita – Consultant. Corprata – Stock Options. Dieta Health – Stock Options. FoodMarble Digestive Health – Consultant, Stock Options. Ironwood Pharmaceuticals – Consultant. Modify Health – Stock Options. Nestlé S.A. – Consultant. Phathom Pharmaceuticals – Consultant. Redhill Biopharma – Consultant. Salix Pharmaceuticals – Consultant. Takeda – Consultant. Vibrant Gastro – Consultant.
Jan Busby-Whitehead indicated no relevant financial relationships.
Marie Gantz indicated no relevant financial relationships.
Heidi Chua indicated no relevant financial relationships.
Stacy Menees indicated no relevant financial relationships.
William Perry indicated no relevant financial relationships.
Holly Richter: Bluewind, Veristat – Advisory Committee/Board Member. COSM – Grant/Research Support. ICA – Consultant. Intl Urogyn J – reimbursement for editor duties. Laborie – Consultant. Neomedic, Moremme – Consultant. Symposia Medicus – CME Speaker. UpToDate – Royalties. WorldWide Fistula Fund, SOLACE – Board of Directors.
Amaanti Sridhar indicated no relevant financial relationships.
Jennifer Wu indicated no relevant financial relationships.
Lawrence Szarka indicated no relevant financial relationships.
Frank Hamilton indicated no relevant financial relationships.
Adil Bharucha, MBBS, MD1, Satish S. C.. Rao, MD, PhD2, William Whitehead, PhD3, Isuzu Meyer, MD, MSPH4, William D. Chey, MD, FACG5, Jan Busby-Whitehead, MD6, Marie Gantz, PhD7, Heidi Chua, MD1, Stacy Menees, MD8, William Perry, MBChB, MPH1, Holly E.. Richter, PhD, MD9, Amaanti Sridhar, MSc7, Jennifer Wu, MD3, Lawrence Szarka, MD1, Frank Hamilton, MD10, 7, Comparative Effectiveness of Biofeedback and Dextranomer/Hyaluronate Injection for Treatment of Fecal Incontinence: A Multicenter Randomized Controlled Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.



