Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 1A: Colorectal Cancer Prevention
13 - Adoption of a Computer-Aided Detection System May Improve Polyp Detection in Patients With Positive Stool-Based Testing
Monday, October 28, 2024
2:35 PM - 2:45 PM ET
Location: Terrace Ballroom 1

Rajesh Keswani, MD, MS
Northwestern Medicine
Chicago, IL
Presenting Author(s)
Rajesh Keswani, MD, MS1, John E.. Pandolfino, MD2
1Northwestern Medicine, Chicago, IL; 2Feinberg School of Medicine, Northwestern University, Chicago, IL
Introduction: Colonoscopy reduces colorectal cancer mortality via the identification and removal of neoplastic polyps. In clinical trials, computer aided detection (CADe) improves polyp detection, but there is limited data of CADe implementation in patients with abnormal stool-based testing. We aimed to assess the impact of CADe upon polyp detection in patients with positive stool-based testing.
Methods: A CADe system (GI Genius, Medtronic) system was implemented in staggered fashion in a single large academic medical center to pragmatically assess its impact over a 9-month period (March 2022 to December 2022). Four CADe units were placed in a twelve-room endoscopy unit where colonoscopists rotate through different rooms. Thus, a colonoscopist may be able to utilize CADe when performing colonoscopy on one day (“CADe room”) but perform colonoscopy in a room without CADe the next day (“non-CADe room”). Colonoscopists were encouraged but not mandated to utilize CADe. For this analysis, the impact of CADe in patients with a positive multitarget stool DNA test (MT-sDNA; Cologuard, Exact Sciences) or fecal immunochemical testing (FIT) was evaluated. The primary outcome was colonoscopy neoplastic (either adenoma or serrated) detection rate (NDR) in patients with abnormal MT-sDNA testing.
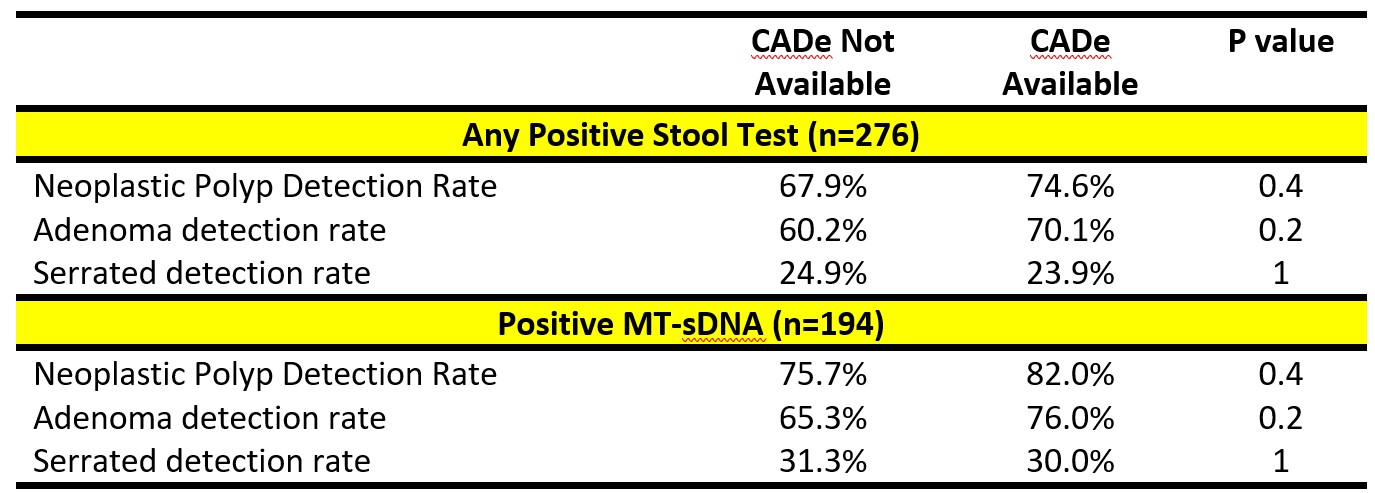
Results: Over a 6-month study period, 51 colonoscopists performed 15,719 colonoscopies of which 194 were performed for positive MT-sDNA testing and 82 for positive FIT testing. Among the patients positive MT-sDNA testing, NDR was non-significantly increased in CADe rooms compared to non-CADe rooms (82% versus 75.7%, p=0.4; Table). Adenoma detection non-significantly increased in CADe rooms as well (76% versus 65.3%, p=0.2). Serrated detection rates were relatively stable between CADe (30%) and non-CADe rooms (31.3%).
For the cohort of 276 patients with any abnormal stool testing (positive MT-sDNA or FIT), NDR (74.6% versus 67.9%, p=0.4) and adenoma detection (70.1% versus 60.3%, p=0.2) non-significantly increased in CADe rooms. Serrated detection did not change in CADe rooms versus non-CADe rooms (23.9% versus 24.9%).
Discussion: In this pragmatic assessment of the impact of CADe upon colonoscopy quality in patients with positive stool testing, CADe non-significantly increased neoplastic polyp detection rates due to improved detection of adenomas. Further work is needed to confirm the benefit of CADe in patients with abnormal stool-based testing.
Disclosures:
Rajesh Keswani, MD, MS1, John E.. Pandolfino, MD2, 13, Adoption of a Computer-Aided Detection System May Improve Polyp Detection in Patients With Positive Stool-Based Testing, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Northwestern Medicine, Chicago, IL; 2Feinberg School of Medicine, Northwestern University, Chicago, IL
Introduction: Colonoscopy reduces colorectal cancer mortality via the identification and removal of neoplastic polyps. In clinical trials, computer aided detection (CADe) improves polyp detection, but there is limited data of CADe implementation in patients with abnormal stool-based testing. We aimed to assess the impact of CADe upon polyp detection in patients with positive stool-based testing.
Methods: A CADe system (GI Genius, Medtronic) system was implemented in staggered fashion in a single large academic medical center to pragmatically assess its impact over a 9-month period (March 2022 to December 2022). Four CADe units were placed in a twelve-room endoscopy unit where colonoscopists rotate through different rooms. Thus, a colonoscopist may be able to utilize CADe when performing colonoscopy on one day (“CADe room”) but perform colonoscopy in a room without CADe the next day (“non-CADe room”). Colonoscopists were encouraged but not mandated to utilize CADe. For this analysis, the impact of CADe in patients with a positive multitarget stool DNA test (MT-sDNA; Cologuard, Exact Sciences) or fecal immunochemical testing (FIT) was evaluated. The primary outcome was colonoscopy neoplastic (either adenoma or serrated) detection rate (NDR) in patients with abnormal MT-sDNA testing.
Results: Over a 6-month study period, 51 colonoscopists performed 15,719 colonoscopies of which 194 were performed for positive MT-sDNA testing and 82 for positive FIT testing. Among the patients positive MT-sDNA testing, NDR was non-significantly increased in CADe rooms compared to non-CADe rooms (82% versus 75.7%, p=0.4; Table). Adenoma detection non-significantly increased in CADe rooms as well (76% versus 65.3%, p=0.2). Serrated detection rates were relatively stable between CADe (30%) and non-CADe rooms (31.3%).
For the cohort of 276 patients with any abnormal stool testing (positive MT-sDNA or FIT), NDR (74.6% versus 67.9%, p=0.4) and adenoma detection (70.1% versus 60.3%, p=0.2) non-significantly increased in CADe rooms. Serrated detection did not change in CADe rooms versus non-CADe rooms (23.9% versus 24.9%).
Discussion: In this pragmatic assessment of the impact of CADe upon colonoscopy quality in patients with positive stool testing, CADe non-significantly increased neoplastic polyp detection rates due to improved detection of adenomas. Further work is needed to confirm the benefit of CADe in patients with abnormal stool-based testing.

Figure: Table 1. Impact of CADe on polyp detection rates in patients with positive stool-based testing
Disclosures:
Rajesh Keswani: Boston Scientific – Consultant. Medtronic – Consultant.
John Pandolfino: Medtronic – Consultant, Intellectual Property/Patents.
Rajesh Keswani, MD, MS1, John E.. Pandolfino, MD2, 13, Adoption of a Computer-Aided Detection System May Improve Polyp Detection in Patients With Positive Stool-Based Testing, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
