Tuesday Poster Session
Category: IBD
P4449 - A Case of an Uncomplicated Pregnancy on Dual Biologic Therapy With Ustekinumab and Vedolizumab for Crohn’s Disease
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
.jpg)
Shayan Amini, MD
Houston Methodist-Weill Cornell Graduate School of Medical Sciences
Houston, TX
Presenting Author(s)
Shayan Amini, MD1, Bincy P. Abraham, MD, MS, FACG2, Kerri Glassner, DO1
1Houston Methodist-Weill Cornell Graduate School of Medical Sciences, Houston, TX; 2Houston Methodist-Weill Cornell, Houston, TX
Introduction: Inflammatory bowel disease (IBD) is associated with increased risk of complications during pregnancy. We report a case of an uncomplicated pregnancy on dual biologic therapy with ustekinumab (UST) and vedolizumab (VDZ) for Crohn’s Disease (CD).
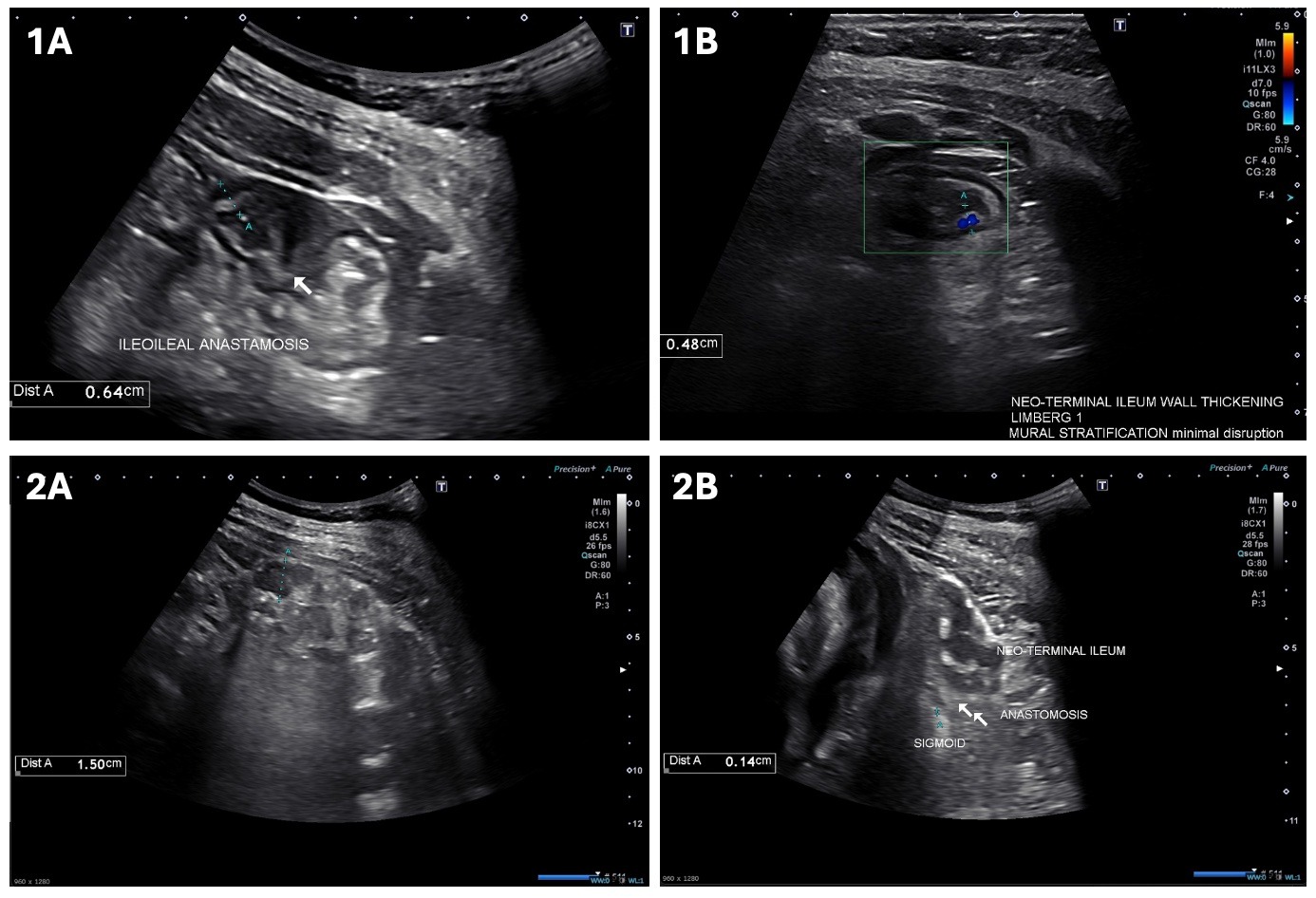
Case Description/Methods: We report the case of a 28-year-old woman with penetrating ileocolonic and perianal CD diagnosed in 2009 who had an uncomplicated pregnancy with normal fetal development while being treated with dual therapy with UST and VDZ. Starting in 2009, she had lack of response to multiple biologics, including infliximab, adalimumab, and certolizumab, requiring ileocolonic resection in 2015. UST was initiated in 2017 due to symptoms and active disease at the neo-terminal ileum on imaging. VDZ was added in 2018 due to persistent symptoms and peri-anal disease requiring fistulectomy and abscess drainage. Despite dual therapy, she had failure to thrive and active disease in the ileum and colon with an SES-CD score of 28 on colonoscopy. She underwent total colectomy with ileorectal anastomosis in 2020. She was continued on both UST and VDZ every 4 weeks due to presence of inflammation (Rutgeerts score i1) in neo-terminal ileum on colonoscopy in 2022. She became pregnant in 2023 when she was clinically stable. Both VDZ and UST were continued every 4 weeks during pregnancy due to intestinal ultrasound (IUS) at 25-week gestation showing mild wall thickening up to 0.64 cm along the ileo-ileal anastomosis with Limberg score 1 on doppler evaluation (Figure 1). Repeat IUS at 35-week gestation showed resolution of prior findings (Figure 2). Patient’s pregnancy was uncomplicated with appropriate fetal development. She underwent cesarean section at 39-weeks gestation without any complications.
Discussion: Pregnancy in IBD is associated with an increased risk for complications such as miscarriage and preterm birth when there is active disease at the time of conception. The PIANO registry demonstrated that the use of immunomodulators (azathioprine, 6-MP), TNF-⍺ inhibitors, UST and VDZ are safe during pregnancy. However, to our knowledge there have not been any previously published cases where dual biologic therapy was continued during pregnancy. Despite having active disease during pregnancy, our patient did well on combination therapy with VDZ and UST with an uncomplicated pregnancy and normal fetal development and outcomes. Additional data is needed to determine the efficacy and safety of dual biologic therapy in certain high-risk patients with IBD.
Disclosures:
Shayan Amini, MD1, Bincy P. Abraham, MD, MS, FACG2, Kerri Glassner, DO1. P4449 - A Case of an Uncomplicated Pregnancy on Dual Biologic Therapy With Ustekinumab and Vedolizumab for Crohn’s Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Houston Methodist-Weill Cornell Graduate School of Medical Sciences, Houston, TX; 2Houston Methodist-Weill Cornell, Houston, TX
Introduction: Inflammatory bowel disease (IBD) is associated with increased risk of complications during pregnancy. We report a case of an uncomplicated pregnancy on dual biologic therapy with ustekinumab (UST) and vedolizumab (VDZ) for Crohn’s Disease (CD).
Case Description/Methods: We report the case of a 28-year-old woman with penetrating ileocolonic and perianal CD diagnosed in 2009 who had an uncomplicated pregnancy with normal fetal development while being treated with dual therapy with UST and VDZ. Starting in 2009, she had lack of response to multiple biologics, including infliximab, adalimumab, and certolizumab, requiring ileocolonic resection in 2015. UST was initiated in 2017 due to symptoms and active disease at the neo-terminal ileum on imaging. VDZ was added in 2018 due to persistent symptoms and peri-anal disease requiring fistulectomy and abscess drainage. Despite dual therapy, she had failure to thrive and active disease in the ileum and colon with an SES-CD score of 28 on colonoscopy. She underwent total colectomy with ileorectal anastomosis in 2020. She was continued on both UST and VDZ every 4 weeks due to presence of inflammation (Rutgeerts score i1) in neo-terminal ileum on colonoscopy in 2022. She became pregnant in 2023 when she was clinically stable. Both VDZ and UST were continued every 4 weeks during pregnancy due to intestinal ultrasound (IUS) at 25-week gestation showing mild wall thickening up to 0.64 cm along the ileo-ileal anastomosis with Limberg score 1 on doppler evaluation (Figure 1). Repeat IUS at 35-week gestation showed resolution of prior findings (Figure 2). Patient’s pregnancy was uncomplicated with appropriate fetal development. She underwent cesarean section at 39-weeks gestation without any complications.
Discussion: Pregnancy in IBD is associated with an increased risk for complications such as miscarriage and preterm birth when there is active disease at the time of conception. The PIANO registry demonstrated that the use of immunomodulators (azathioprine, 6-MP), TNF-⍺ inhibitors, UST and VDZ are safe during pregnancy. However, to our knowledge there have not been any previously published cases where dual biologic therapy was continued during pregnancy. Despite having active disease during pregnancy, our patient did well on combination therapy with VDZ and UST with an uncomplicated pregnancy and normal fetal development and outcomes. Additional data is needed to determine the efficacy and safety of dual biologic therapy in certain high-risk patients with IBD.

Figure: Figure 1: Intestinal ultrasound showing wall thickening around the site of ileo-ileal anastomosis and Limberg 1 score on Doppler evaluation.
Figure 2: Normal ileo-ileal anastomosis and ileo-colonic anastomosis.
Figure 2: Normal ileo-ileal anastomosis and ileo-colonic anastomosis.
Disclosures:
Shayan Amini indicated no relevant financial relationships.
Bincy P. Abraham: AbbVie – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Janssen – Consultant, Speakers Bureau. Medtronic – Consultant. Pfizer – Consultant, Speakers Bureau. Prometheus – Consultant. Samsung Bioepis – Consultant. Takeda – Consultant, Speakers Bureau.
Kerri Glassner: Eli Lilly – Advisor or Review Panel Member. Janssen – Advisor or Review Panel Member. Pfizer – Advisor or Review Panel Member, Speakers Bureau.
Shayan Amini, MD1, Bincy P. Abraham, MD, MS, FACG2, Kerri Glassner, DO1. P4449 - A Case of an Uncomplicated Pregnancy on Dual Biologic Therapy With Ustekinumab and Vedolizumab for Crohn’s Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
