Tuesday Poster Session
Category: Biliary/Pancreas
P3461 - Mitigating Financial Toxicity of Pancreatic Enzyme Replacement Therapy in Medicare Part D
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
- CB
Christopher A. Bouvette, MD
University of Oklahoma College of Medicine
Oklahoma City, OK
Presenting Author(s)
Christopher A. Bouvette, MD1, Ijlal Akbar Ali, MD1, Amir Rumman, MD1, Abdul-Sattar Shariq Mohammed, MD2, Muhammad K. Hasan, MD2, Mustafa Arain, MD2
1University of Oklahoma College of Medicine, Oklahoma City, OK; 2AdventHealth, Orlando, FL
Introduction: Pancreatic enzyme replacement therapy (PERT) plays a vital role in improving clinical outcomes and quality of life for patients with chronic pancreatitis. However, once patients on Medicare Part D (MPD) reach a certain prescription threshold, they enter a coverage gap (donut hole) wherein they have high out-of-pocket cost until reaching catastrophic coverage. Alternative sourcing of PERT and the new Inflation Reduction Act (IRA) effective year 2025 may, in part, mitigate some of these out-of-pocket costs. We aimed to explore the potential impact of the IRA on PERT associated cost for Medicare patients.
Methods: We queried 2022 Medicare Part D (MPD) data by prescriber to identify gastroenterology-specific claims for pancrealipase formulations; Creon, Zenpep, Pancreaze, Pertzye, and Viokace. A cost analysis at alternative supplier price points (Amazon, GoodRx, and Veteran’s Health Association National Formulary) was then performed on Medicare-wide Part D 2022 claims. Good Rx price-point was determined as an average across five zip-code limited searches (New York City, Los Angeles, Chicago, Houston, and Phoenix). Finally, based on 2022 MPD average spending per beneficiary, we modeled cost of Creon under the current MPD and tentative IRA structure.
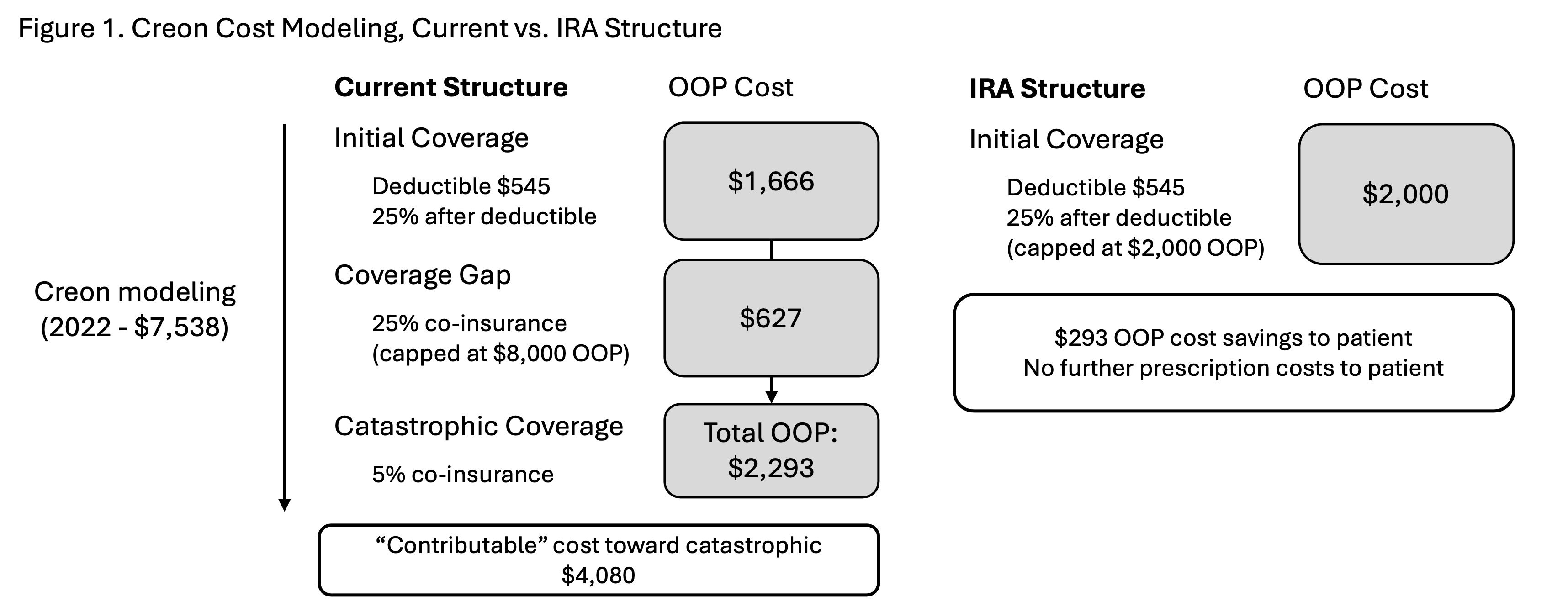
Results: Total MPD PERT spending was $1.67 billion; Creon spending was $1.31 billion (78%). Alternative supply at Amazon and GoodRx price-point was constitutively unfavorable, mean relative cost 1.34 and 1.38 respectively. Zenpep was also unfavorable at VHA price-point; although others yielded potential cost savings. (Table 1) Modeled under current MPD structure an annual supply of Creon falls within the coverage gap and results in ongoing additional medication costs to patients. Under the tentative IRA structure, patients accumulated maximum out-of-pocket cost with PERT alone and would lead to no additional annual prescription cost for patients. (Figure 1)
Discussion: We found that PERT translates to significant financial burden on MPD participants. Annual claims for PERT alone places patients within the coverage gap, with only minor progression towards catastrophic coverage. A favorable VHA price-point implies that if permitted, federal price negotiation may translate to potential cost savings (approximately $10 million annually). Finally, modeling under the tentative IRA structure demonstrates a new horizon, with limits to out-of-pocket costs and particular benefit in those patients with any accessory claims.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Christopher A. Bouvette, MD1, Ijlal Akbar Ali, MD1, Amir Rumman, MD1, Abdul-Sattar Shariq Mohammed, MD2, Muhammad K. Hasan, MD2, Mustafa Arain, MD2. P3461 - Mitigating Financial Toxicity of Pancreatic Enzyme Replacement Therapy in Medicare Part D, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Oklahoma College of Medicine, Oklahoma City, OK; 2AdventHealth, Orlando, FL
Introduction: Pancreatic enzyme replacement therapy (PERT) plays a vital role in improving clinical outcomes and quality of life for patients with chronic pancreatitis. However, once patients on Medicare Part D (MPD) reach a certain prescription threshold, they enter a coverage gap (donut hole) wherein they have high out-of-pocket cost until reaching catastrophic coverage. Alternative sourcing of PERT and the new Inflation Reduction Act (IRA) effective year 2025 may, in part, mitigate some of these out-of-pocket costs. We aimed to explore the potential impact of the IRA on PERT associated cost for Medicare patients.
Methods: We queried 2022 Medicare Part D (MPD) data by prescriber to identify gastroenterology-specific claims for pancrealipase formulations; Creon, Zenpep, Pancreaze, Pertzye, and Viokace. A cost analysis at alternative supplier price points (Amazon, GoodRx, and Veteran’s Health Association National Formulary) was then performed on Medicare-wide Part D 2022 claims. Good Rx price-point was determined as an average across five zip-code limited searches (New York City, Los Angeles, Chicago, Houston, and Phoenix). Finally, based on 2022 MPD average spending per beneficiary, we modeled cost of Creon under the current MPD and tentative IRA structure.
Results: Total MPD PERT spending was $1.67 billion; Creon spending was $1.31 billion (78%). Alternative supply at Amazon and GoodRx price-point was constitutively unfavorable, mean relative cost 1.34 and 1.38 respectively. Zenpep was also unfavorable at VHA price-point; although others yielded potential cost savings. (Table 1) Modeled under current MPD structure an annual supply of Creon falls within the coverage gap and results in ongoing additional medication costs to patients. Under the tentative IRA structure, patients accumulated maximum out-of-pocket cost with PERT alone and would lead to no additional annual prescription cost for patients. (Figure 1)
Discussion: We found that PERT translates to significant financial burden on MPD participants. Annual claims for PERT alone places patients within the coverage gap, with only minor progression towards catastrophic coverage. A favorable VHA price-point implies that if permitted, federal price negotiation may translate to potential cost savings (approximately $10 million annually). Finally, modeling under the tentative IRA structure demonstrates a new horizon, with limits to out-of-pocket costs and particular benefit in those patients with any accessory claims.

Figure: Figure 1
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Christopher Bouvette indicated no relevant financial relationships.
Ijlal Akbar Ali indicated no relevant financial relationships.
Amir Rumman indicated no relevant financial relationships.
Abdul-Sattar Shariq Mohammed indicated no relevant financial relationships.
Muhammad Hasan: Boston Scientific – Consultant. microtech – Consultant. Neptune Medical – Consultant. Olympus – Consultant.
Mustafa Arain indicated no relevant financial relationships.
Christopher A. Bouvette, MD1, Ijlal Akbar Ali, MD1, Amir Rumman, MD1, Abdul-Sattar Shariq Mohammed, MD2, Muhammad K. Hasan, MD2, Mustafa Arain, MD2. P3461 - Mitigating Financial Toxicity of Pancreatic Enzyme Replacement Therapy in Medicare Part D, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

