Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 3A - Esophagus / Stomach / Practice Management
45 - Impact of Elafibranor on Markers of Disease Activity and Progression in Patients With Advanced Primary Biliary Cholangitis (Late-Breaking Abstract)
Tuesday, October 29, 2024
3:35 PM - 3:45 PM ET
Location: Terrace Ballroom 1

Marlyn J. Mayo, MD, FACG (she/her/hers)
Professor
UT Southwestern Medical Center
Dallas, TX
Late Breaking Abstract Presenter(s)
Marlyn J. Mayo, MD, FACG,1 Kris V. Kowdley, MD, FACG,2 Christopher L. Bowlus, MD,3 John M. Vierling, MD,4 Ira M. Jacobson, MD, FACG,5 Nuno Antunes, PhD,6 Marwan Sleiman, PhD,7 Nathan Touati, MSc,7 Claudia O. Zein, MD,6 Cynthia Levy, MD8; 1University of Texas Southwestern Medical Center, Dallas, TX, 2Liver Institute Northwest, Seattle, WA, 3UC Davis School of Medicine, Sacramento, CA, 4Baylor College of Medicine, Houston, TX, 5NYU Langone Health, New York, NY, 6Ipsen, Cambridge, MA, 7Ipsen, Boulogne-Billancourt, Île-de-France, France, 8Schiff Center for Liver Diseases, University of Miami, Miami, FL
Introduction: In the phase III ELATIVE trial (NCT04526665), elafibranor treatment resulted in significant biochemical response in patients with primary biliary cholangitis (PBC). Here, we evaluate treatment response stratified by baseline disease stage, focusing on advanced stage.
Methods: PBC disease stage was categorized as early or advanced based on liver stiffness measurement (LSM; ≤10 or >10 kPa), or histology (Nakanuma fibrosis score <2 or >2, indicating absent/mild fibrosis or advanced fibrosis/cirrhosis) among patients who had a liver biopsy. Biochemical response at Week 52 was defined as alkaline phosphatase (ALP) <1.67 times upper limit of normal, with a >15% reduction from baseline, and normal total bilirubin (TB), via the Cochran-Mantel-Haenszel method, imputing non response for patients who experienced intercurrent events. Changes from baseline to Week 52 for ALP, TB, albumin (ALB), alanine aminotransferase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT), and LSM were analyzed via mixed models for repeated measures according to baseline disease stage.
Results: Of the 161 patients enrolled in ELATIVE, 54 had advanced stage PBC (elafibranor n=35; placebo n=19) and 107 had early stage (elafibranor n=73; placebo n=34). Mean baseline values of ALP, TB, ALT, AST, GGT, and LSM were higher in patients with advanced stage than in early stage for both treatment arms. Among patients with early stage PBC, biochemical response at Week 52 was achieved in 53.4% of patients receiving elafibranor and in 5.9% receiving placebo. Among those with advanced stage PBC, biochemical response occurred in 45.7% of patients receiving elafibranor and 0% receiving placebo. Advanced versus early risk difference was –0.016 (95% confidence interval [CI]: –0.216, 0.184); elafibranor versus placebo risk difference was 0.457 (95% CI: 0.230, 0.618) for advanced disease. Reductions in ALP, ALT, AST, and GGT levels were observed among those with advanced stage PBC receiving elafibranor, with TB, LSM, and ALB remaining relatively unchanged. In patients with advanced stage receiving placebo, ALP, ALT, and AST levels remained stable, while TB and LSM levels showed trends for an increase, and ALB for a decrease (Figure).
Discussion: In ELATIVE, elafibranor treatment provided a consistent treatment effect regardless of disease stage. In patients with advanced stage PBC, the improvement in surrogate liver biomarkers observed with elafibranor treatment suggest a positive impact on disease stabilization.
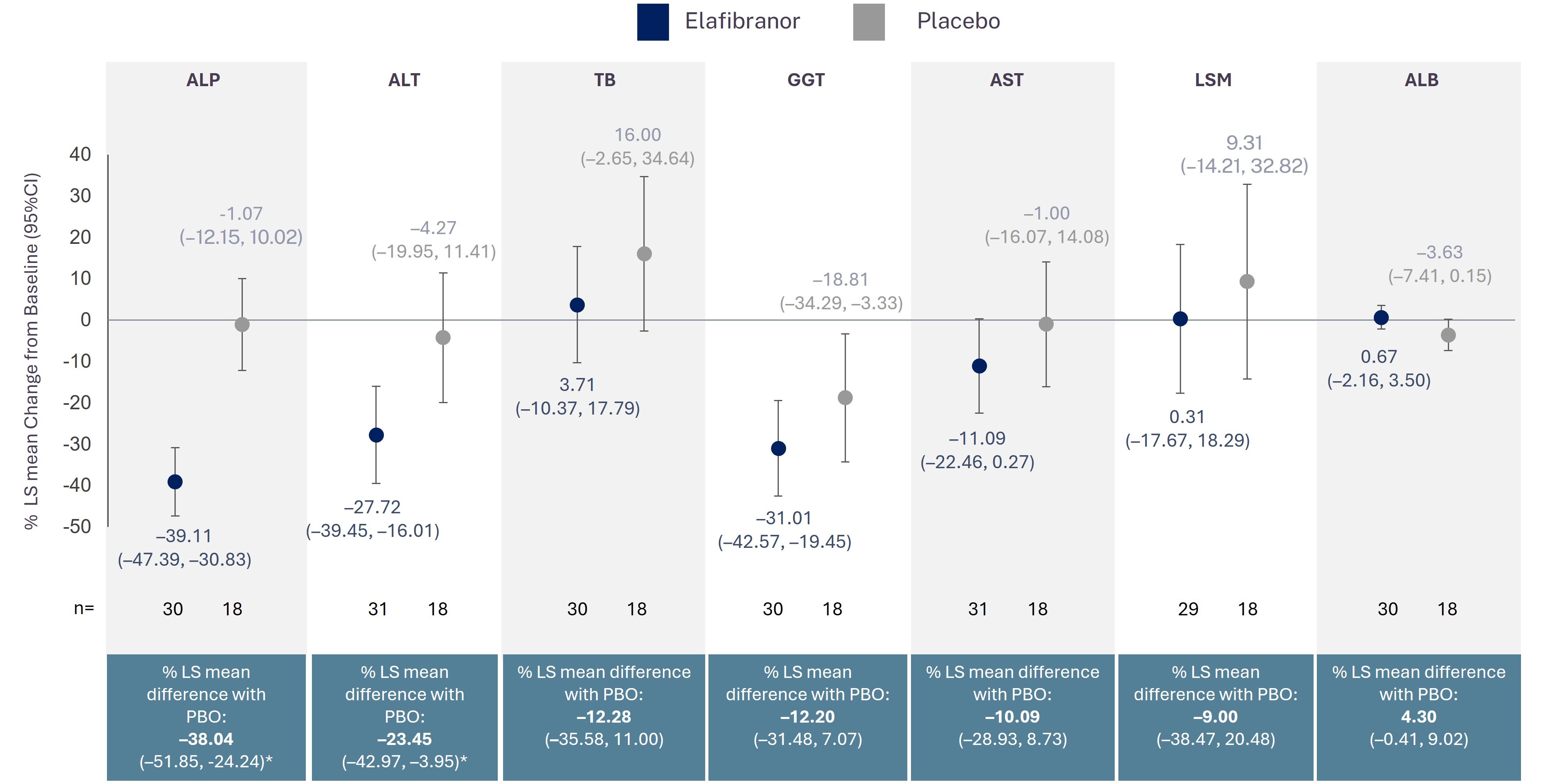
Figure: Percentage change from baseline to Week 52 in key markers of disease activity in patients with advanced stage PBC in ELATIVE
*p<0.05. Data are presented as LS mean percentage change from baseline (95% CI) for elafibranor and placebo, and percent LS mean difference (95% CI). Analysis uses the mixed model repeated measures with treatment, visits (until week 52) and treatment-by-visit interaction as fixed factors and adjusting for baseline values and the stratification factors. Baseline is defined as the last non-missing central value on or before the first dose of the randomized study medication. The data observed one day or more after the occurrence of an ICE (study treatment discontinuation or use of rescue therapy for PBC) have been considered as missing data and have been handled within the model itself (MAR assumption). Missing data not related to ICE are also handled within the model itself (MAR assumption). ALB: albumin; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CI: confidence interval; GGT: gamma-glutamyl transferase; ICE: intercurrent event; LS: least squares; LSM: liver stiffness measurement; MAR: missing at random; PBO: placebo; TB: total bilirubin.
*p<0.05. Data are presented as LS mean percentage change from baseline (95% CI) for elafibranor and placebo, and percent LS mean difference (95% CI). Analysis uses the mixed model repeated measures with treatment, visits (until week 52) and treatment-by-visit interaction as fixed factors and adjusting for baseline values and the stratification factors. Baseline is defined as the last non-missing central value on or before the first dose of the randomized study medication. The data observed one day or more after the occurrence of an ICE (study treatment discontinuation or use of rescue therapy for PBC) have been considered as missing data and have been handled within the model itself (MAR assumption). Missing data not related to ICE are also handled within the model itself (MAR assumption). ALB: albumin; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CI: confidence interval; GGT: gamma-glutamyl transferase; ICE: intercurrent event; LS: least squares; LSM: liver stiffness measurement; MAR: missing at random; PBO: placebo; TB: total bilirubin.
Disclosures: Kris Kowdley: Received grants from Boston Scientific, Corcept, CymaBay, GENFIT, Gilead, GlaxoSmithKline, Hanmi, Intercept, Ipsen, Janssen, Madrigal, Mirum, Novo Nordisk, NGM Biopharmaceuticals, Pfizer, Pliant Therapeutics, Terns, Viking Therapeutics, Zydus, and 89bio Inc.; Received royalties or licenses from UpToDate; Received consulting fees from CymaBay, Enanta, GENFIT, Gilead, HighTide, Inipharm, Intercept, Ipsen, Madrigal, Mirum, NGM Biopharmaceuticals, Pliant, Pfizer, Protagonist, Zydus, and 89bio Inc.; Received payment or honoraria from AbbVie, Gilead, and Intercept; Received payment for expert testimony from the Department of Justice; Participant on a Data Safety Monitoring Board or Advisory Board for CTI, Medpace, Labcorp, and Worldwide Clinical Trials; Stockholder in Inipharm; Receipt of equipment, materials, drugs, medical writing, gifts or other services from Velacur; Christopher Bowlus: Received grants from Calliditas, Chemomab, COUR, Cymabay, Gilead, GlaxoSmithKline, Hanmi, Intercept, Ipsen, Mirum, Novartis, Novo Nordisk, Pliant Therapeutics, Viking Therapeutics, and Zydus; Received consulting fees from Chemomab, Cymabay, GlaxoSmithKline, Ipsen, Mirum, NGM Biopharmaceuticals, and Pliant Therapeutics; Marlyn Mayo: Received grants from CymaBay, GENFIT, Gilead, GlaxoSmithKline, Ironwood, Intercept, Ipsen, and Mirum; Received consulting fees from CymaBay, GlaxoSmithKline, Intra-Sana, Ipsen and Mallinckrodt; Received support for attending meetings and/or travel from CymaBay, Ipsen, and Mallinckrodt; John Vierling: Received grants from CymaBay, Eli Lilly, Escient, GENFIT, Genkyotex, Gilead, Ipsen, Intercept, NGM Biopharmaceuticals, Novartis, Roche-Genentech, and Zydus; Scientific advisor for Arena, Blade, CymaBay, Gilead, Intercept, Ipsen, Kezar, Lilly, Moderna, Novartis, Parvus, Perspectum, and Roche-Genentech; Ira Jacobson: Received grants from AstraZeneca, AusperBio, CymaBay, Eli Lilly, Gilead, GlaxoSmithKline, Inventiva, Intercept, Ipsen, Madrigal, Merck, Mirum, and Novo Nordisk; Received consulting fees from Arbutus, CymaBay, Gilead, GlaxoSmithKline, Intercept, Madrigal, Merck, Moderna, and Precision Biosciences; Participated in a Data Monitoring Committee for Aligos, Altimmune, GlaxoSmithKline, and Takeda; Nuno Antunes, Marwan Sleiman: Employees and shareholders of Ipsen; Nathan Touati: Employee of Ipsen; Claudia Zein: Employee and shareholder of Ipsen at the time of this analysis; Cynthia Levy: Received grants from Calliditas, CymaBay, Escient, Gilead, GlaxoSmithKline, Intercept, Ipsen, Kowa, Mirum, Target RWE and Zydus; Received consulting fees from Calliditas, CymaBay, GlaxoSmithKline, Gilead, Intercept, Ipsen, Kowa and Mirum; Participation on a Data Safety Monitoring Board for COUR; Associate Editor of Hepatology.
Marlyn J. Mayo, MD, FACG, Kris V. Kowdley, MD, FACG, Christopher L. Bowlus, MD, John M. Vierling, MD, Ira M. Jacobson, MD, FACG, Nuno Antunes, PhD, Marwan Sleiman, PhD, Nathan Touati, MSc, Claudia O. Zein, MD, Cynthia Levy, MD, 45, Impact of Elafibranor on Markers of Disease Activity and Progression in Patients With Advanced Primary Biliary Cholangitis (late-breaking abstract), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

