Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4A - Liver
62 - GLP-1RA and Safety of Gastrointestinal (GI) Endoscopic Procedures A Systematic Review and Meta-Analysis (Late-Breaking Abstract)
Wednesday, October 30, 2024
9:40 AM - 9:50 AM ET
Location: Terrace Ballroom 1

Violeta B. Popov, MD, PhD, FACG
Director of Bariatric Endoscopy
NYU Langone Health NY VA Harbor Health System (Manhattan)
New York, NY
Late Breaking Abstract Presenter(s)
Violeta Popov, MD, PhD, FACG1, Mohammad Bilal, MD2, Ali M. Ahmed, MD3, Aaron Yeoh, MD4, Christen Dilly, MD5, Marianna Papademetriou, MD6, Heiko Pohl, MD7, Fadi Antaki, MD8, Jason A. Dominitz, MD, MHS9; 1NY VA Harbor Healthcare System, New York, NY, 2Minneapolis VA Medical Center, Minneapolis, MN, 3Birmingham VA Medical Center, Birmingham, AL, 4Palo Alto VA Medical Center, Palo Alto, CA, 5Richard L. Roudebush VA Medical Center, Indianapolis, IN, 6Washington DC VA Medical Center, Washington, DC, 7White River Junction VA Medical Center, White River Junction, VT, 8John D. Dingell VA Medical Center, Detroit, MI, 9VA Puget Sound Health Care, Seattle, WA
Introduction: GLP-1RA are increasingly used to treat type 2 diabetes mellitus and obesity. Their potential to slow gastric emptying has raised safety concerns for GI endoscopy. In 2023, the American Society of Anesthesiology(ASA) recommended holding GLP-1RA before GI procedures, although an increased risk of aspiration due to GLP-1RA had not been established. We conducted a systematic review and meta-analysis to evaluate the impact of GLP-1RA on these outcomes of G endoscopy.
Methods: MEDLINE, Embase, and Cochrane Database were searched through July 1, 2024. Primary outcomes included the pooled rates and odds ratios (OR) with 95% confidence intervals (CI) of aspiration/aspiration pneumonia (AP) and aborted upper endoscopy (aEGD) in patients on GLP-1RA undergoing EGD and EGD/colonoscopy compared to patients who were not on GLP-1RA. Secondary outcomes included the pooled rate and OR of retained gastric contents (RGC).
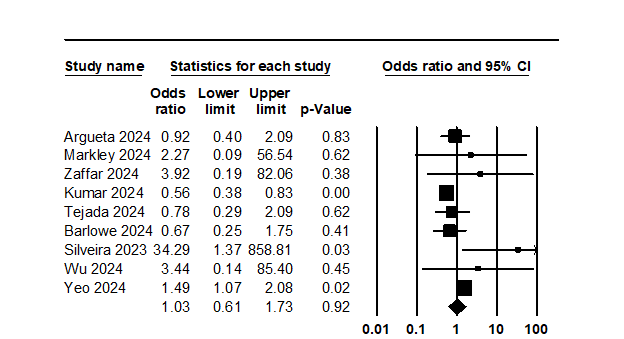
Results: Out of 264 citations, 35 studies (13 published papers, 22 conference abstracts) with 203,216 study and 511,093 control subjects were included in our analysis. Most studies (25) were retrospective single-center studies, 4 were multicenter studies, and 6 used large databases. Most studies used the ASA publication time as a cut-off to define the GLP-1RA and non-GLP-1RA cohorts. Primary outcomes: The OR (95% CI) for AP after EGD with GLP-1RA use was 1.03 (0.61, 1.73, Figure 1). AP was reported in 0.3% (0.12%, 0.87%) of GLP-1RA users and 0.23% (0.1%, 0.67%) of non-GLP-1RA users. The OR for aEGD in GLP-1RA users was 5.19 (3.5, 7.70), with aEGD reported for 1.9% (1.4%, 2.7%) in GLP-1RA users and 0.4% (0.2%, 1.0%) in non-GLP-1RA users. Secondary Outcomes: The OR for RGC in GLP-1RA was 4.38 (3.14, 6.1) after EGD, with a rate of 12.5% (10.0%, 15.4%) in GLP-1 users and 3.4% (2.2%, 5.0%) in non-GLP-1RA users. The rate of RGC in GLP-1RA users undergoing both EGD and colonoscopy was 5.5% (3.6%, 8.2%).
Conclusion: Our data suggest that GLP-1RA are not associated with an increased risk of aspiration in patients who have not held the medication prior to EGD. However, there is a higher rate of aborted EGDs and RGC in patients receiving GLP-1RAs. This increased RGC risk was not observed in patients undergoing simultaneous EGD and colonoscopy, suggesting that clear liquid diet and bowel preparation may mitigate this outcome. Professional societies should consider recommending a 24-hour clear liquid diet prior to GI endoscopy without discontinuing GLP-1RA therapy.
Disclosures: Violeta Popov: Microtech Endoscopy, Research grant; Ali Ahmed: Boston Scientific, Pentax, Consultant; All other authors have indicated no relevant financial relationships.
Violeta Popov, MD, PhD, FACG, Mohammad Bilal, MD, Ali M. Ahmed, MD, Aaron Yeoh, MD, Christen Dilly, MD, Marianna Papademetriou, MD, Heiko Pohl, MD, Fadi Antaki, MD, Jason A. Dominitz, MD, MHS, 62, Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RA) and Safety of Gastrointestinal (GI) Endoscopic Procedures: A Systematic Review and Meta-Analysis (late-breaking abstract), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Introduction: GLP-1RA are increasingly used to treat type 2 diabetes mellitus and obesity. Their potential to slow gastric emptying has raised safety concerns for GI endoscopy. In 2023, the American Society of Anesthesiology(ASA) recommended holding GLP-1RA before GI procedures, although an increased risk of aspiration due to GLP-1RA had not been established. We conducted a systematic review and meta-analysis to evaluate the impact of GLP-1RA on these outcomes of G endoscopy.
Methods: MEDLINE, Embase, and Cochrane Database were searched through July 1, 2024. Primary outcomes included the pooled rates and odds ratios (OR) with 95% confidence intervals (CI) of aspiration/aspiration pneumonia (AP) and aborted upper endoscopy (aEGD) in patients on GLP-1RA undergoing EGD and EGD/colonoscopy compared to patients who were not on GLP-1RA. Secondary outcomes included the pooled rate and OR of retained gastric contents (RGC).
Results: Out of 264 citations, 35 studies (13 published papers, 22 conference abstracts) with 203,216 study and 511,093 control subjects were included in our analysis. Most studies (25) were retrospective single-center studies, 4 were multicenter studies, and 6 used large databases. Most studies used the ASA publication time as a cut-off to define the GLP-1RA and non-GLP-1RA cohorts. Primary outcomes: The OR (95% CI) for AP after EGD with GLP-1RA use was 1.03 (0.61, 1.73, Figure 1). AP was reported in 0.3% (0.12%, 0.87%) of GLP-1RA users and 0.23% (0.1%, 0.67%) of non-GLP-1RA users. The OR for aEGD in GLP-1RA users was 5.19 (3.5, 7.70), with aEGD reported for 1.9% (1.4%, 2.7%) in GLP-1RA users and 0.4% (0.2%, 1.0%) in non-GLP-1RA users. Secondary Outcomes: The OR for RGC in GLP-1RA was 4.38 (3.14, 6.1) after EGD, with a rate of 12.5% (10.0%, 15.4%) in GLP-1 users and 3.4% (2.2%, 5.0%) in non-GLP-1RA users. The rate of RGC in GLP-1RA users undergoing both EGD and colonoscopy was 5.5% (3.6%, 8.2%).
Conclusion: Our data suggest that GLP-1RA are not associated with an increased risk of aspiration in patients who have not held the medication prior to EGD. However, there is a higher rate of aborted EGDs and RGC in patients receiving GLP-1RAs. This increased RGC risk was not observed in patients undergoing simultaneous EGD and colonoscopy, suggesting that clear liquid diet and bowel preparation may mitigate this outcome. Professional societies should consider recommending a 24-hour clear liquid diet prior to GI endoscopy without discontinuing GLP-1RA therapy.

Figure: Odds Ratio (OR) for aspiration in GLP-1RA users vs. non-GLP-1RA users undergoing EGD
Disclosures: Violeta Popov: Microtech Endoscopy, Research grant; Ali Ahmed: Boston Scientific, Pentax, Consultant; All other authors have indicated no relevant financial relationships.
Violeta Popov, MD, PhD, FACG, Mohammad Bilal, MD, Ali M. Ahmed, MD, Aaron Yeoh, MD, Christen Dilly, MD, Marianna Papademetriou, MD, Heiko Pohl, MD, Fadi Antaki, MD, Jason A. Dominitz, MD, MHS, 62, Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RA) and Safety of Gastrointestinal (GI) Endoscopic Procedures: A Systematic Review and Meta-Analysis (late-breaking abstract), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

