Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 2B - Colon / General Endoscopy / GI Bleed / Practice Management
33 - Glucagon-Like Peptide-1 Receptor Agonists and Capsule Endoscopy in Patients with Diabetes: A Matched Cohort Study
Tuesday, October 29, 2024
9:20 AM - 9:30 AM ET
Location: Terrace Ballroom 2-3
.jpeg.jpg)
Tarek Odah, MD
Mayo Clinic
Jacksonville, FL
Presenting Author(s)
Award: ACG Governors Award for Excellence in Clinical Research
Tarek Odah, MD, Asrita Vattikonda, MD, Mark Stark, MD, Bhaumik Brahmbhatt, MD, Frank J. Lukens, MD, Dilhana S. Badurdeen, MD, Jana G. Hashash, MD, MSc, Francis A. Farraye, MD, MSc
Mayo Clinic, Jacksonville, FL
Introduction: Video capsule endoscopy (VCE) is valuable for assessing conditions like gastrointestinal bleeding, anemia, and inflammatory bowel disease. Glucagon-like peptide-1 receptor agonists (GLP-1 RA) are commonly prescribed for diabetes management and, more recently, for weight loss, with their pharmacologic effects including a delay in gastric emptying. This study investigates the impact of GLP-1 RA usage on VCE outcomes in patients with diabetes.
Methods: This retrospective matched cohort study focuses on patients with diabetes undergoing VCE while receiving GLP-1 RA. The control group consisted of patients with diabetes not using GLP-1 RA, matched for demographics and diabetes-related factors at a 1:1 ratio. The primary outcome is gastric transit time in VCE studies, with secondary outcome being incomplete small bowel evaluation and the small bowel transit time.
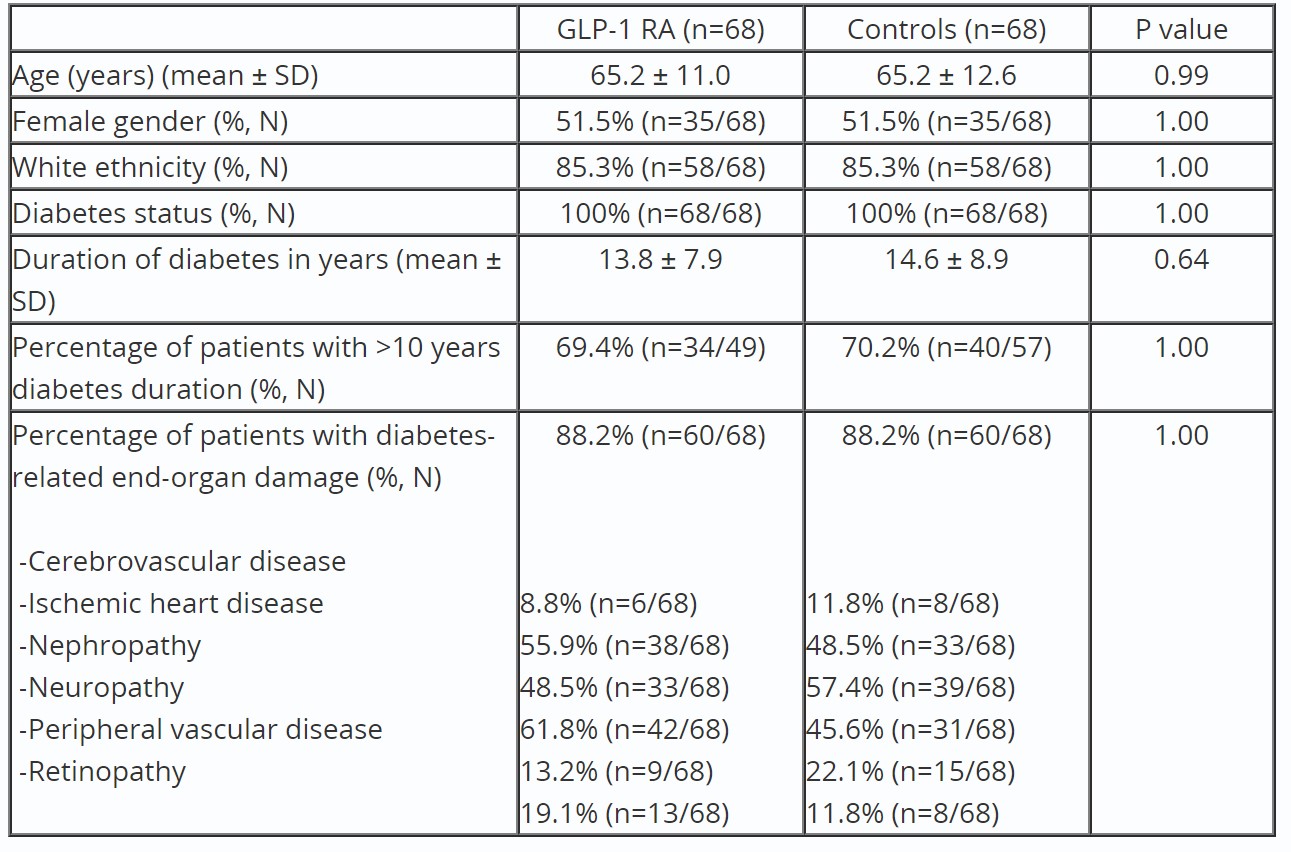
Results: Baseline demographics and diabetes related characteristics of patients on GLP-1 RA and controls are shown in Table 1. In the 68 GLP-1 RA patient cohort, five (7%) experienced VCE failure to pass through the stomach, while all controls passed successfully (p=0.06). GLP-1 RA patients had longer gastric transit time (99.3 ± 134.2 minutes) compared to controls (25.3 ± 31.6 minutes, p < 0.001). Multivariate analysis revealed GLP-1 RA usage increased gastric transit time by 79.4 minutes (CI: 37.8-121.0, p < 0.001) compared to controls after adjusting on relevant factors. Furthermore, sixteen GLP-1 RA patients (23.5%) experienced incomplete passage of the VCE through the small intestine, a significantly higher rate compared to three patients in the control group (4.4%) (p< 0.01). value=< 0.01). The average small intestine transit time was 262.5 minutes ± 100.0, slightly longer than the matched controlled cohort’s average of 237.8 ± 85.5 (p value=0.16).
Discussion: GLP-1 RA usage prolongs gastric transit time and increases incomplete small bowel evaluation during VCE. Future studies may be crucial for evaluating strategies to mitigate these effects.
Disclosures:
Tarek Odah, MD, Asrita Vattikonda, MD, Mark Stark, MD, Bhaumik Brahmbhatt, MD, Frank J. Lukens, MD, Dilhana S. Badurdeen, MD, Jana G. Hashash, MD, MSc, Francis A. Farraye, MD, MSc, 33, Glucagon-Like Peptide-1 Receptor Agonists and Capsule Endoscopy in Patients with Diabetes: A Matched Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Tarek Odah, MD, Asrita Vattikonda, MD, Mark Stark, MD, Bhaumik Brahmbhatt, MD, Frank J. Lukens, MD, Dilhana S. Badurdeen, MD, Jana G. Hashash, MD, MSc, Francis A. Farraye, MD, MSc
Mayo Clinic, Jacksonville, FL
Introduction: Video capsule endoscopy (VCE) is valuable for assessing conditions like gastrointestinal bleeding, anemia, and inflammatory bowel disease. Glucagon-like peptide-1 receptor agonists (GLP-1 RA) are commonly prescribed for diabetes management and, more recently, for weight loss, with their pharmacologic effects including a delay in gastric emptying. This study investigates the impact of GLP-1 RA usage on VCE outcomes in patients with diabetes.
Methods: This retrospective matched cohort study focuses on patients with diabetes undergoing VCE while receiving GLP-1 RA. The control group consisted of patients with diabetes not using GLP-1 RA, matched for demographics and diabetes-related factors at a 1:1 ratio. The primary outcome is gastric transit time in VCE studies, with secondary outcome being incomplete small bowel evaluation and the small bowel transit time.
Results: Baseline demographics and diabetes related characteristics of patients on GLP-1 RA and controls are shown in Table 1. In the 68 GLP-1 RA patient cohort, five (7%) experienced VCE failure to pass through the stomach, while all controls passed successfully (p=0.06). GLP-1 RA patients had longer gastric transit time (99.3 ± 134.2 minutes) compared to controls (25.3 ± 31.6 minutes, p < 0.001). Multivariate analysis revealed GLP-1 RA usage increased gastric transit time by 79.4 minutes (CI: 37.8-121.0, p < 0.001) compared to controls after adjusting on relevant factors. Furthermore, sixteen GLP-1 RA patients (23.5%) experienced incomplete passage of the VCE through the small intestine, a significantly higher rate compared to three patients in the control group (4.4%) (p< 0.01). value=< 0.01). The average small intestine transit time was 262.5 minutes ± 100.0, slightly longer than the matched controlled cohort’s average of 237.8 ± 85.5 (p value=0.16).
Discussion: GLP-1 RA usage prolongs gastric transit time and increases incomplete small bowel evaluation during VCE. Future studies may be crucial for evaluating strategies to mitigate these effects.

Table: Title:
Table 1: Baseline demographics and diabetes related characteristics of patients on GLP-1 RA and controls
Table 1: Baseline demographics and diabetes related characteristics of patients on GLP-1 RA and controls
Disclosures:
Tarek Odah indicated no relevant financial relationships.
Asrita Vattikonda indicated no relevant financial relationships.
Mark Stark indicated no relevant financial relationships.
Bhaumik Brahmbhatt indicated no relevant financial relationships.
Frank J. Lukens indicated no relevant financial relationships.
Dilhana Badurdeen indicated no relevant financial relationships.
Jana Hashash: Bristol Myers Squibb – Consultant.
Francis Farraye: AbbVie – Consultant. Avalo Therapeutics – Consultant. Bausch – Advisor or Review Panel Member. BMS – Consultant. Braintree Labs – Consultant. DSMB for Lilly. – Sits on. Fresenius Kabi – Consultant. GI Reviewers and IBD Educational Group – independent contractor. GSK, Iterative Health, Janssen, Pfizer, Pharmacosmos, Sandoz Immunology, Sebela and Viatris – Consultant.
Tarek Odah, MD, Asrita Vattikonda, MD, Mark Stark, MD, Bhaumik Brahmbhatt, MD, Frank J. Lukens, MD, Dilhana S. Badurdeen, MD, Jana G. Hashash, MD, MSc, Francis A. Farraye, MD, MSc, 33, Glucagon-Like Peptide-1 Receptor Agonists and Capsule Endoscopy in Patients with Diabetes: A Matched Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.



