Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 3A - Esophagus / Stomach / Practice Management
37 - GLP-1 Agonists Use Is Associated With Lower Use of PPI and Do Not Worsen Outcomes in Patients With GERD: A National Database Analysis
Tuesday, October 29, 2024
2:15 PM - 2:25 PM ET
Location: Terrace Ballroom 1

Luis M. Nieto, MD (he/him/his)
Emory University School of Medicine
Atlanta, GA
Presenting Author(s)
Award: ACG Auxiliary Award (Trainee)
Luis M.. Nieto, MD1, Sharon Narvaez, MD2, Pedro Palacios-Argueta, MD3, Do Han Kim, MD4, Donghyun Ko, MD5, Saurabh Chawla, MD1, Kenneth Vega, MD, FACG6, Laura Davisson, MD7
1Emory University School of Medicine, Atlanta, GA; 2Universidad de Guayaquil, School of Medicine, Atlanta, GA; 3Mayo Clinic Florida, Jacksonville, FL; 4Mount Sinai Morningside and West, Icahn School of Medicine at Mount Sinai, New York, NY; 5Yale-New Haven Health/Bridgeport Hospital, Bridgeport, CT; 6Medical College of Georgia at Augusta University, Augusta, GA; 7West Virginia University, Morgantown, WV
Introduction: Gastroesophageal reflux disease (GERD) is one of the most common gastrointestinal conditions with an estimated prevalence between 6%-30% in the United States. Use of Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has been shown to increase development of GERD. However, there is a paucity of data related to the impact of starting GLP-1 RAs in patients with history of GERD. Therefore, we aim to evaluate GERD outcomes in patients who start taking those drugs.
Methods: We performed a retrospective cohort study utilizing large population-based data from the TriNetX platform. We identified patients older than 18 with history of GERD who started GLP-1 RAs (semaglutide, liraglutide, dulaglutide and tirzepatide) between January 1, 2010, and December 31, 2023. This cohort was matched with patients with history of GERD who did not receive GLP-1 RAs according to age, demographics, comorbidities, and medication by using 1:1 propensity matching. The primary endpoint was all-cause mortality, and the secondary endpoints were GERD complications (includes Barrett’s esophagus, esophagitis, strictures, and new onset asthma), esophageal cancer, diagnostic procedures need (includes EGD and manometry), fundoplication, and Proton Pump Inhibitors (PPI) use. Logistic regression was used to estimate odd ratios (ORs).
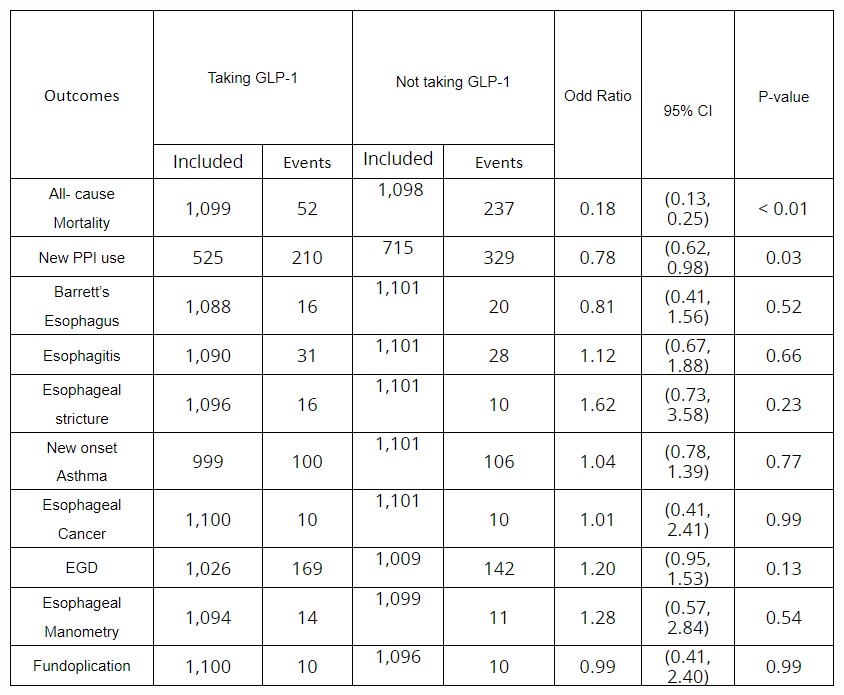
Results: A total of 273,629 adult patients with history of GERD without complications were identified, 2,760 of these individuals received GLP -1 RAs at some point since January 1, 2010; 1,101 out of 2,760 (mean [SD] age, 55.2 [12.3] years; 685 [62.21%] female) were matched with 1,101 individuals (mean [SD] age, 55.5 [14.4] years; 665 [60.40%] female) who did not received GLP-1 RAs. See table 1-2. The GLP-1 RAs group had significant lower odds of all-cause mortality (ORs, 0.18; 95% Confidence Interval [CI], 0.13-0.25) and lower odds of new PPI use (ORs, 0.78; 95% CI, 0.62-0.98). No differences were found in the incidence of complications, esophageal cancer, and needs for procedures or surgery.
Discussion: In this retrospective study of national database, we found that patients with history of GERD who start taking GLP-1 RAs have lower odds of all-cause mortality and new use of PPI. Also, showed that use of GLP-1 RAs is not associated with worsening of GERD outcomes. Further prospective studies are needed to determine the mechanism for these clinical findings.
Disclosures:
Luis M.. Nieto, MD1, Sharon Narvaez, MD2, Pedro Palacios-Argueta, MD3, Do Han Kim, MD4, Donghyun Ko, MD5, Saurabh Chawla, MD1, Kenneth Vega, MD, FACG6, Laura Davisson, MD7, 37, GLP-1 Agonists Use Is Associated With Lower Use of PPI and Do Not Worsen Outcomes in Patients With GERD: A National Database Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Luis M.. Nieto, MD1, Sharon Narvaez, MD2, Pedro Palacios-Argueta, MD3, Do Han Kim, MD4, Donghyun Ko, MD5, Saurabh Chawla, MD1, Kenneth Vega, MD, FACG6, Laura Davisson, MD7
1Emory University School of Medicine, Atlanta, GA; 2Universidad de Guayaquil, School of Medicine, Atlanta, GA; 3Mayo Clinic Florida, Jacksonville, FL; 4Mount Sinai Morningside and West, Icahn School of Medicine at Mount Sinai, New York, NY; 5Yale-New Haven Health/Bridgeport Hospital, Bridgeport, CT; 6Medical College of Georgia at Augusta University, Augusta, GA; 7West Virginia University, Morgantown, WV
Introduction: Gastroesophageal reflux disease (GERD) is one of the most common gastrointestinal conditions with an estimated prevalence between 6%-30% in the United States. Use of Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has been shown to increase development of GERD. However, there is a paucity of data related to the impact of starting GLP-1 RAs in patients with history of GERD. Therefore, we aim to evaluate GERD outcomes in patients who start taking those drugs.
Methods: We performed a retrospective cohort study utilizing large population-based data from the TriNetX platform. We identified patients older than 18 with history of GERD who started GLP-1 RAs (semaglutide, liraglutide, dulaglutide and tirzepatide) between January 1, 2010, and December 31, 2023. This cohort was matched with patients with history of GERD who did not receive GLP-1 RAs according to age, demographics, comorbidities, and medication by using 1:1 propensity matching. The primary endpoint was all-cause mortality, and the secondary endpoints were GERD complications (includes Barrett’s esophagus, esophagitis, strictures, and new onset asthma), esophageal cancer, diagnostic procedures need (includes EGD and manometry), fundoplication, and Proton Pump Inhibitors (PPI) use. Logistic regression was used to estimate odd ratios (ORs).
Results: A total of 273,629 adult patients with history of GERD without complications were identified, 2,760 of these individuals received GLP -1 RAs at some point since January 1, 2010; 1,101 out of 2,760 (mean [SD] age, 55.2 [12.3] years; 685 [62.21%] female) were matched with 1,101 individuals (mean [SD] age, 55.5 [14.4] years; 665 [60.40%] female) who did not received GLP-1 RAs. See table 1-2. The GLP-1 RAs group had significant lower odds of all-cause mortality (ORs, 0.18; 95% Confidence Interval [CI], 0.13-0.25) and lower odds of new PPI use (ORs, 0.78; 95% CI, 0.62-0.98). No differences were found in the incidence of complications, esophageal cancer, and needs for procedures or surgery.
Discussion: In this retrospective study of national database, we found that patients with history of GERD who start taking GLP-1 RAs have lower odds of all-cause mortality and new use of PPI. Also, showed that use of GLP-1 RAs is not associated with worsening of GERD outcomes. Further prospective studies are needed to determine the mechanism for these clinical findings.

Table: Primary and Secondary Outcomes in patients with GERD who are taking GLP-1 RAs and not taking GLP-1 RAs
Disclosures:
Luis Nieto indicated no relevant financial relationships.
Sharon Narvaez indicated no relevant financial relationships.
Pedro Palacios-Argueta indicated no relevant financial relationships.
Do Han Kim indicated no relevant financial relationships.
Donghyun Ko indicated no relevant financial relationships.
Saurabh Chawla indicated no relevant financial relationships.
Kenneth Vega indicated no relevant financial relationships.
Laura Davisson indicated no relevant financial relationships.
Luis M.. Nieto, MD1, Sharon Narvaez, MD2, Pedro Palacios-Argueta, MD3, Do Han Kim, MD4, Donghyun Ko, MD5, Saurabh Chawla, MD1, Kenneth Vega, MD, FACG6, Laura Davisson, MD7, 37, GLP-1 Agonists Use Is Associated With Lower Use of PPI and Do Not Worsen Outcomes in Patients With GERD: A National Database Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


