Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 3A - Esophagus / Stomach / Practice Management
40 - High Economic Burden Yet Low Clinical Value: Why Is So Much Sucralfate Prescribed?
Tuesday, October 29, 2024
2:45 PM - 2:55 PM ET
Location: Terrace Ballroom 1
- NW
Neha Wadhavkar, MD
Brown University
Providence, RI
Presenting Author(s)
Neha Wadhavkar, MD1, Laura Varnum, PharmD2, Steven Moss, MD3
1Brown University, Providence, RI; 2Brown Medicine/Brown Physicians, Inc., Providence, RI; 3Brown Medicine/Lifespan, Providence, RI
Introduction: Sucralfate is a commonly prescribed medication in use for decades. Sucralfate is thought to act as a mucosal protectant via aluminum salt binding, with no effect on acid secretion. Sucralfate received FDA approval in 1981 for short-term treatment of duodenal ulcers, though with much less evidence than was subsequently shown for proton pump inhibitors (PPI) or histamine-2 receptor antagonists (H2RA). Sucralfate’s package inserts reference trials of superiority to placebo in ulcer healing, though methodologic details are lacking. Few randomized controlled trials of sucralfate exist. Its role in gastric ulcers, non-ulcer dyspepsia, and reflux esophagitis is uncertain. Recent ACG guidelines recommend against sucralfate for reflux disease. We review the financial impact of prescribing sucralfate.
Methods: We examined the most recent publicly available pharmacologic data on the ClinCalc database to investigate trends in utilization and aggregate costs for sucralfate in comparison to other medications for upper gastrointestinal conditions.
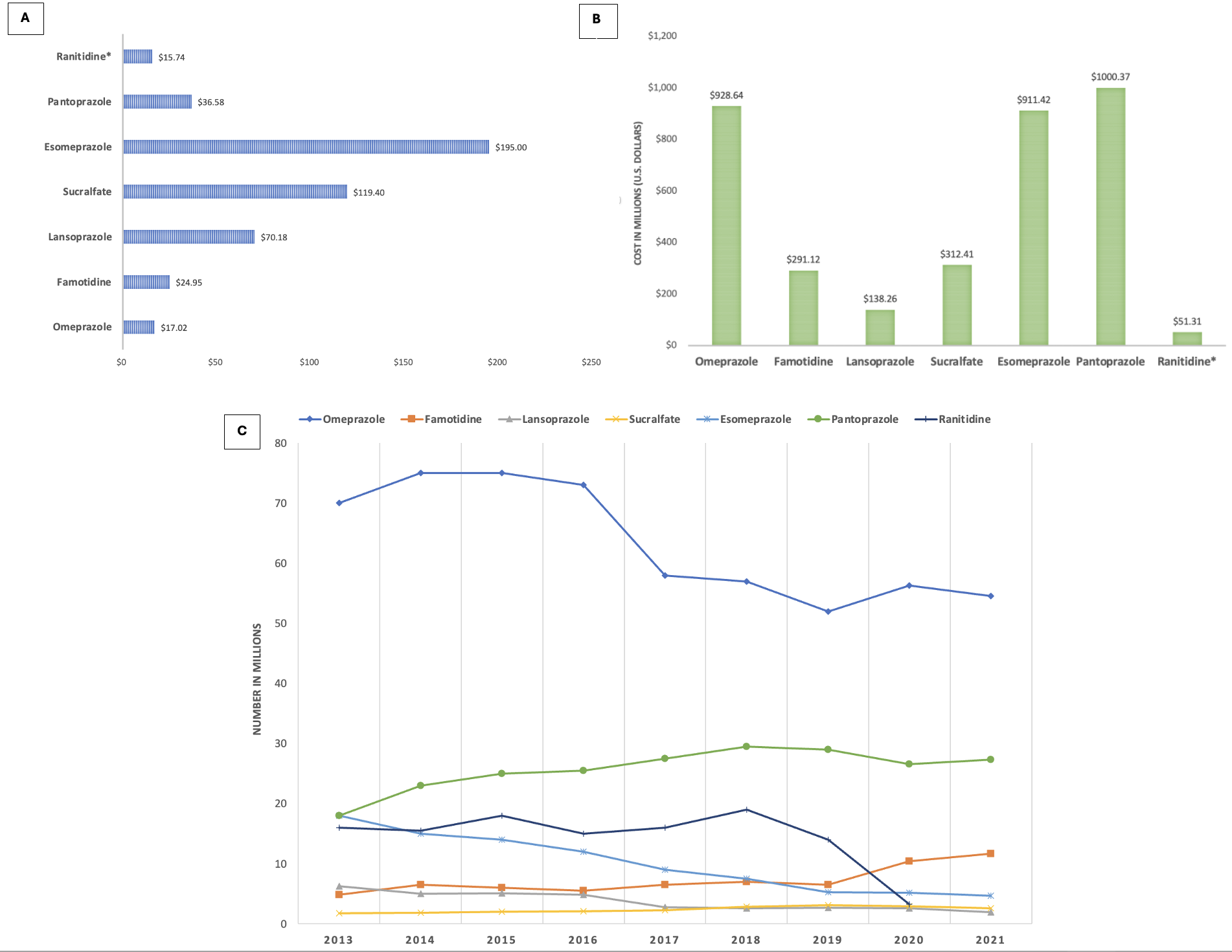
Results: Sucralfate was second behind esomeprazole for most expensive prescription ($119 versus $195), followed by lansoprazole at $70 (Fig 1A). Least expensive were ranitidine ($16) and omeprazole ($17). Most PPIs had a high total annual cost, pantoprazole over $1 billion and both omeprazole and esomeprazole over $900 million (Fig 1B). The annual cost of sucralfate was $312 million, higher than famotidine, lansoprazole, or ranitidine. From 2013 to 2021, the number of sucralfate prescriptions rose from 1.8 million to 2.6 million (Fig 1C). Famotidine prescriptions increased from 4.9 million to 11.7 million. Omeprazole remained the most prescribed, though from 2015 to 2021, its use declined from 75 million to 54 million.
Discussion: Sucralfate expenditure is comparable to PPIs and H2RAs, despite the paucity of clinical data justifying its often off-label usage. Unlike PPIs and H2RA, sucralfate is not approved for reflux or dyspepsia, even as adjunct therapy. Concerns over ranitidine’s carcinogenic effects led to its recall, leaving famotidine as the primary H2RA. We suspect that PPI usage will continue to decline given recent media attention over possible adverse effects. In this vacuum, more patients and providers appear to be turning to sucralfate. Appropriate randomized clinical trials are warranted to justify this trend. Until then, we should question our colleagues’ continual prescription of an expensive and poorly studied old-fashioned cure-all.
Disclosures:
Neha Wadhavkar, MD1, Laura Varnum, PharmD2, Steven Moss, MD3, 40, High Economic Burden Yet Low Clinical Value: Why Is So Much Sucralfate Prescribed?, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Brown University, Providence, RI; 2Brown Medicine/Brown Physicians, Inc., Providence, RI; 3Brown Medicine/Lifespan, Providence, RI
Introduction: Sucralfate is a commonly prescribed medication in use for decades. Sucralfate is thought to act as a mucosal protectant via aluminum salt binding, with no effect on acid secretion. Sucralfate received FDA approval in 1981 for short-term treatment of duodenal ulcers, though with much less evidence than was subsequently shown for proton pump inhibitors (PPI) or histamine-2 receptor antagonists (H2RA). Sucralfate’s package inserts reference trials of superiority to placebo in ulcer healing, though methodologic details are lacking. Few randomized controlled trials of sucralfate exist. Its role in gastric ulcers, non-ulcer dyspepsia, and reflux esophagitis is uncertain. Recent ACG guidelines recommend against sucralfate for reflux disease. We review the financial impact of prescribing sucralfate.
Methods: We examined the most recent publicly available pharmacologic data on the ClinCalc database to investigate trends in utilization and aggregate costs for sucralfate in comparison to other medications for upper gastrointestinal conditions.
Results: Sucralfate was second behind esomeprazole for most expensive prescription ($119 versus $195), followed by lansoprazole at $70 (Fig 1A). Least expensive were ranitidine ($16) and omeprazole ($17). Most PPIs had a high total annual cost, pantoprazole over $1 billion and both omeprazole and esomeprazole over $900 million (Fig 1B). The annual cost of sucralfate was $312 million, higher than famotidine, lansoprazole, or ranitidine. From 2013 to 2021, the number of sucralfate prescriptions rose from 1.8 million to 2.6 million (Fig 1C). Famotidine prescriptions increased from 4.9 million to 11.7 million. Omeprazole remained the most prescribed, though from 2015 to 2021, its use declined from 75 million to 54 million.
Discussion: Sucralfate expenditure is comparable to PPIs and H2RAs, despite the paucity of clinical data justifying its often off-label usage. Unlike PPIs and H2RA, sucralfate is not approved for reflux or dyspepsia, even as adjunct therapy. Concerns over ranitidine’s carcinogenic effects led to its recall, leaving famotidine as the primary H2RA. We suspect that PPI usage will continue to decline given recent media attention over possible adverse effects. In this vacuum, more patients and providers appear to be turning to sucralfate. Appropriate randomized clinical trials are warranted to justify this trend. Until then, we should question our colleagues’ continual prescription of an expensive and poorly studied old-fashioned cure-all.

Figure: Figure 1A. Cost per prescription for common gastroenterological medications in 2021.
Figure 1B. Total annual cost of common gastroenterological medications in 2021.
Figure 1C. Total number of prescriptions for common gastroenterological medications from 2013 to 2021.
*For ranitidine, only data up to 2020 available due to its recall.
Figure 1B. Total annual cost of common gastroenterological medications in 2021.
Figure 1C. Total number of prescriptions for common gastroenterological medications from 2013 to 2021.
*For ranitidine, only data up to 2020 available due to its recall.
Disclosures:
Neha Wadhavkar indicated no relevant financial relationships.
Laura Varnum indicated no relevant financial relationships.
Steven Moss indicated no relevant financial relationships.
Neha Wadhavkar, MD1, Laura Varnum, PharmD2, Steven Moss, MD3, 40, High Economic Burden Yet Low Clinical Value: Why Is So Much Sucralfate Prescribed?, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

