Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 3B - Obesity / Biliary-Pancreas
51 - Safety and Efficacy of Endoscopic Ultrasound-Guided Radiofrequency Ablation for Branch Duct-Intraductal Papillary Mucinous Neoplasms: A Single Arm Clinical Trial
Tuesday, October 29, 2024
3:05 PM - 3:15 PM ET
Location: Terrace Ballroom 2-3
- VP
Vanisha Patel, MD
The Ohio State University Wexner Medical Center
Columbus, OH
Presenting Author(s)
Award: ACG Governors Award for Excellence in Clinical Research (Trainee)
Vanisha Patel, MD1, Zarine K. Shah, MD1, Phil Hart, MD1, Stacey Culp, PhD2, Hamza Shah, MD1, Raj Shah, MD1, Jordan Burlen, MD1, Peter J Lee, MD1, Georgios Papachristou, MD, PhD1, Somashekar G Krishna, MD1
1The Ohio State University Wexner Medical Center, Columbus, OH; 2Ohio State University, Columbus, OH
Introduction: Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) is a novel, non-surgical approach for treating pancreatic lesions in patients with high-surgical risk. This trial aims to evaluate the safety and efficacy of EUS-RFA for the thermal ablation of branch duct-intraductal papillary mucinous neoplasms (BD-IPMN).
Methods: Non-operative patients with BD-IPMNs meeting surgical resection criteria underwent EUS-RFA in a single-arm trial (NCT05961982). EUS-guided confocal endomicroscopy and cyst fluid next-generation sequencing (NGS) were used for diagnosis and risk stratification. EUS-RFA was performed with a 19G needle (TaeWoong Medical USA) in Continuance Mode on the VIVA™ Combo System (STARMed, South Korea) applying 50W energy doses until ablation completion. Pre- and post-RFA cyst volume estimates with magnetic resonance imaging and NGS fluid mutation changes were analyzed to identify predictors of volume change.
Results: Twenty-three participants (mean age 75±7 years; 6 female) with 27 BD-IPMNs (mean diameter 4.6±1.7 cm) underwent EUS-RFA. Each BD-IPMN received a median of 16 applications during one session of EUS-RFA. Five participants received a second EUS-RFA treatment at 3-6 months following index treatment.
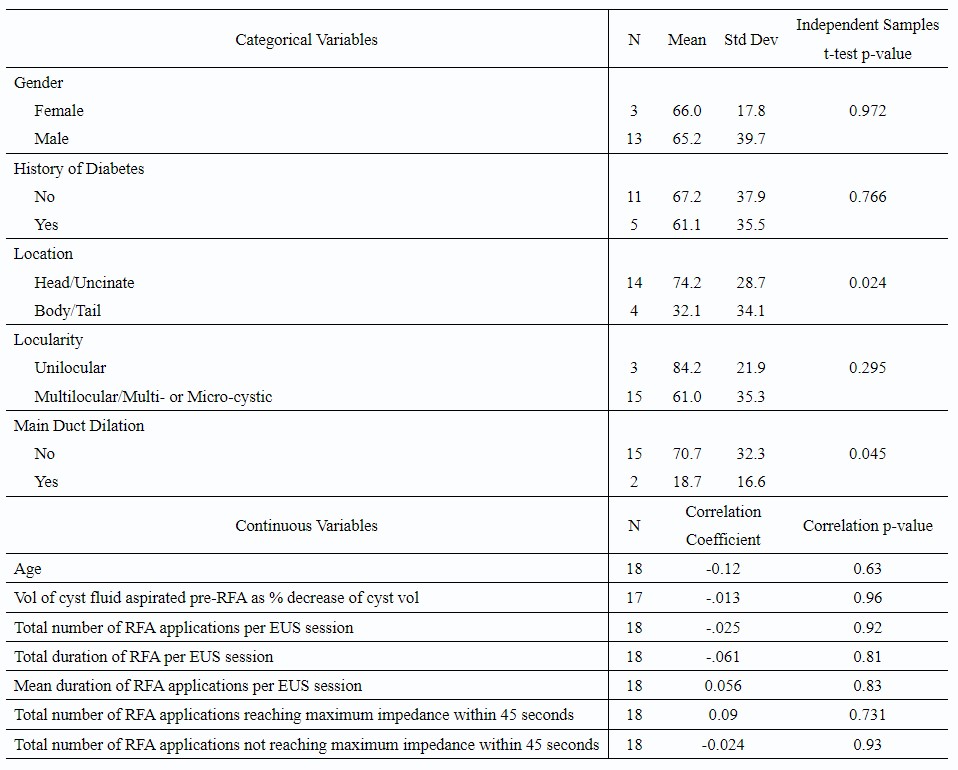
Post EUS-RFA follow-up was available for 18 BD-IPMNs (n=16 patients) over a mean duration of 6±4 months. A median volume reduction of 70% (23.2 ml to 3.9 ml; p< 0.001) was observed. Complete ablation occurred in 17% (n=3) and more than 50% volume reduction in 78% (n=14). BD-IPMN location in the head/uncinate (p=0.02) and lack of main duct dilation (p=0.04) were significantly associated with post-RFA percentage decrease in volume.
Follow-up cyst fluid NGS demonstrated an absence of KRAS/GNAS mutations in 33% (n=4/12). In 12 participants, a 72% mean decrease in KRAS variant allele fraction (mean of 23.3±17.0% to 6.5±21.3%, p=0.03) was noted.
Adverse events occurred in 9% of EUS-RFA sessions involving 3 participants. These events included bleeding, duodenal and biliary stricture, acute pancreatitis, and duodenal perforation; all were successfully managed.
Discussion: This first prospective trial of EUS-RFA for BD-IPMNs demonstrates a high proportion of clinically significant responses in cyst volume and KRAS mutation burden with acceptable morbidity in a high-risk patient population. Ongoing work is needed to determine the most appropriate measures of treatment efficacy and durability of response.
Disclosures:
Vanisha Patel, MD1, Zarine K. Shah, MD1, Phil Hart, MD1, Stacey Culp, PhD2, Hamza Shah, MD1, Raj Shah, MD1, Jordan Burlen, MD1, Peter J Lee, MD1, Georgios Papachristou, MD, PhD1, Somashekar G Krishna, MD1, 51, Safety and Efficacy of Endoscopic Ultrasound-Guided Radiofrequency Ablation for Branch Duct-Intraductal Papillary Mucinous Neoplasms: A Single Arm Clinical Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Vanisha Patel, MD1, Zarine K. Shah, MD1, Phil Hart, MD1, Stacey Culp, PhD2, Hamza Shah, MD1, Raj Shah, MD1, Jordan Burlen, MD1, Peter J Lee, MD1, Georgios Papachristou, MD, PhD1, Somashekar G Krishna, MD1
1The Ohio State University Wexner Medical Center, Columbus, OH; 2Ohio State University, Columbus, OH
Introduction: Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) is a novel, non-surgical approach for treating pancreatic lesions in patients with high-surgical risk. This trial aims to evaluate the safety and efficacy of EUS-RFA for the thermal ablation of branch duct-intraductal papillary mucinous neoplasms (BD-IPMN).
Methods: Non-operative patients with BD-IPMNs meeting surgical resection criteria underwent EUS-RFA in a single-arm trial (NCT05961982). EUS-guided confocal endomicroscopy and cyst fluid next-generation sequencing (NGS) were used for diagnosis and risk stratification. EUS-RFA was performed with a 19G needle (TaeWoong Medical USA) in Continuance Mode on the VIVA™ Combo System (STARMed, South Korea) applying 50W energy doses until ablation completion. Pre- and post-RFA cyst volume estimates with magnetic resonance imaging and NGS fluid mutation changes were analyzed to identify predictors of volume change.
Results: Twenty-three participants (mean age 75±7 years; 6 female) with 27 BD-IPMNs (mean diameter 4.6±1.7 cm) underwent EUS-RFA. Each BD-IPMN received a median of 16 applications during one session of EUS-RFA. Five participants received a second EUS-RFA treatment at 3-6 months following index treatment.
Post EUS-RFA follow-up was available for 18 BD-IPMNs (n=16 patients) over a mean duration of 6±4 months. A median volume reduction of 70% (23.2 ml to 3.9 ml; p< 0.001) was observed. Complete ablation occurred in 17% (n=3) and more than 50% volume reduction in 78% (n=14). BD-IPMN location in the head/uncinate (p=0.02) and lack of main duct dilation (p=0.04) were significantly associated with post-RFA percentage decrease in volume.
Follow-up cyst fluid NGS demonstrated an absence of KRAS/GNAS mutations in 33% (n=4/12). In 12 participants, a 72% mean decrease in KRAS variant allele fraction (mean of 23.3±17.0% to 6.5±21.3%, p=0.03) was noted.
Adverse events occurred in 9% of EUS-RFA sessions involving 3 participants. These events included bleeding, duodenal and biliary stricture, acute pancreatitis, and duodenal perforation; all were successfully managed.
Discussion: This first prospective trial of EUS-RFA for BD-IPMNs demonstrates a high proportion of clinically significant responses in cyst volume and KRAS mutation burden with acceptable morbidity in a high-risk patient population. Ongoing work is needed to determine the most appropriate measures of treatment efficacy and durability of response.

Table: Percent Decrease in Volume of Branch Duct-Intraductal Papillary Mucinous Neoplasm Following Endoscopic Ultrasound-guided Radiofrequency Ablation
Disclosures:
Vanisha Patel indicated no relevant financial relationships.
Zarine Shah indicated no relevant financial relationships.
Phil Hart indicated no relevant financial relationships.
Stacey Culp indicated no relevant financial relationships.
Hamza Shah indicated no relevant financial relationships.
Raj Shah indicated no relevant financial relationships.
Jordan Burlen indicated no relevant financial relationships.
Peter J Lee indicated no relevant financial relationships.
Georgios Papachristou indicated no relevant financial relationships.
Somashekar G Krishna: Boston Scientific – Consultant. Mauna Kea Technologies, and TaeWoong Medical USA – Grant/Research Support. TaeWoong Medical USA – Grant/Research Support.
Vanisha Patel, MD1, Zarine K. Shah, MD1, Phil Hart, MD1, Stacey Culp, PhD2, Hamza Shah, MD1, Raj Shah, MD1, Jordan Burlen, MD1, Peter J Lee, MD1, Georgios Papachristou, MD, PhD1, Somashekar G Krishna, MD1, 51, Safety and Efficacy of Endoscopic Ultrasound-Guided Radiofrequency Ablation for Branch Duct-Intraductal Papillary Mucinous Neoplasms: A Single Arm Clinical Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.



