Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4B - IBD
65 - Fecal Microbiota Transplantation (FMT) With Bezlotoxumab Was Not Better Than FMT Alone in Inflammatory Bowel Disease Patients and Recurrent Clostridioides difficile Infection Results from a Randomized Controlled Trial
Wednesday, October 30, 2024
8:30 AM - 8:40 AM ET
Location: Terrace Ballroom 2-3

Jessica R. Allegretti, MD, MPH, FACG
Director, Crohn's and Colitis Center, Brigham and Women's Hospital; Associate Professor of Medicine
Harvard Medical School
Cambridge, MA
Presenting Author(s)
Award: ACG Governors Award for Excellence in Clinical Research
Jessica R. Allegretti, MD, MPH, FACG1, Jordan Axelrad, MD, MPH2, Rahul S. Dalal, MD, MPH3, Heidy Cabral, BS, MS3, Alexander Carlin, BS3, Jenna Marcus, BA4, Colleen R. Kelly, MD, FACG4, Ari Grinspan, MD5, Monika Fischer, MD, MS6
1Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 2NYU Grossman School of Medicine, New York, NY; 3Brigham and Women's Hospital, Boston, MA; 4Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6Indiana University, Indianapolis, IN
Introduction: Fecal microbiota transplantation (FMT) has been shown to be safe and effective among inflammatory bowel disease (IBD) patients with recurrent Clostridioides difficile infection (rCDI). Additionally, bezlotoxumab, a fully human monoclonal antibody that binds to C. difficile toxin B, is indicated to prevent recurrence of CDI in adults at risk for rCDI. We aimed to assess the impact of FMT in combination with bezlotoxumab in patients with IBD and rCDI.
Methods: Multicenter randomized placebo-controlled trial. Patients with IBD and 2 or more episodes of CDI received a colonoscopic FMT. Patients were randomized 1:1 to receive a single bezlotoxumab infusion or placebo prior to the FMT. CDI testing (GDH/EIA toxin and PCR) was performed pre-FMT, and at 1, 8, and 12 weeks post-FMT. The primary outcome was CDI recurrence up to week 8 defined as diarrhea and EIA+ toxin test. The secondary outcome assessed was CDI decolonization post-treatment through week 12 defined as negative PCR testing through week 12. Risk factors for ongoing colonization at week 12 were assessed using univariate logistic regression.
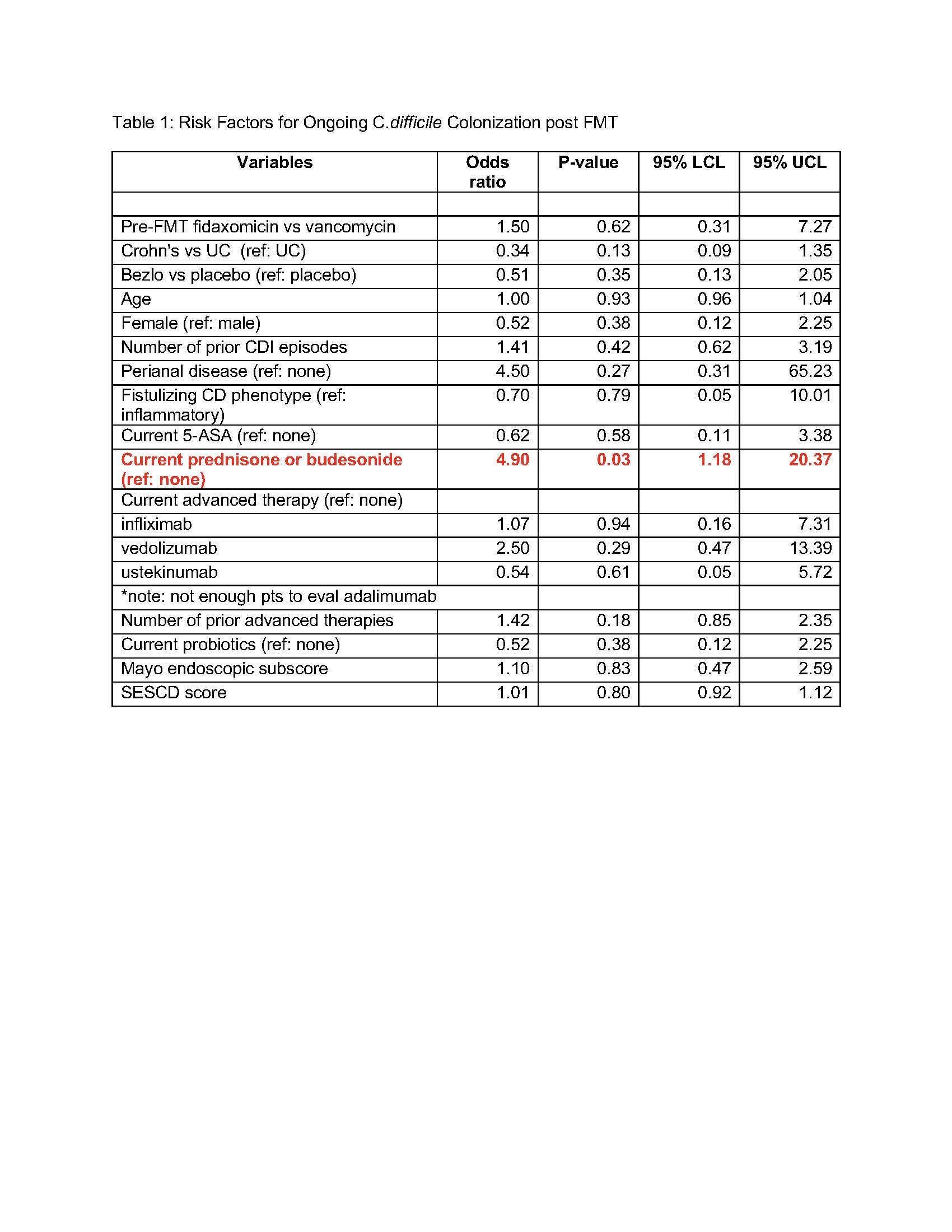
Results: 61 participants were enrolled with a median age of 38 (IQR 28,54), 54% were male (n=33), 20 of which had Crohn’s disease (CD) (mean HBI =7.2) and 41 had ulcerative colitis (UC) (mean Partial Mayo Score =3.2). Median baseline fecal calprotectin was 635 (IQR 150, 2615). Thirty patients were in the treatment arm and 31 in the placebo arm, with no significant differences between groups. 5 participants (8%) experienced a CDI recurrence with confirmed EIA+ stool, of these 4 were in the treatment arm and 1 was in the placebo arm (13% vs 3%, p=0.15). With regards to C.difficile colonization, though not statistically significant, more patients in the treatment arm were decolonized compared to placebo at week 1 (82% vs 68%, p=0.22) and at week 12 (83% vs 72%, p=0.34). Risk factors for ongoing colonization were assessed (Table 1). Steroid use at the time of FMT led to a significant increased risk of ongoing colonization of C.difficile at week 12 post FMT (OR: 4.90, 95% CI: 1.18-20.37, p=0.03).
Discussion: This is the first clinical trial to assess the effect of FMT in combination with bezlotoxumab in patients with IBD and rCDI. The data suggests no clear efficacy benefit to a combination approach. Steroid use at the time of FMT was a significant risk factor for ongoing colonization 12 weeks post FMT, a critical factor for future risk of recurrence.
Disclosures:
Jessica R. Allegretti, MD, MPH, FACG1, Jordan Axelrad, MD, MPH2, Rahul S. Dalal, MD, MPH3, Heidy Cabral, BS, MS3, Alexander Carlin, BS3, Jenna Marcus, BA4, Colleen R. Kelly, MD, FACG4, Ari Grinspan, MD5, Monika Fischer, MD, MS6, 65, Fecal Microbiota Transplantation (FMT) With Bezlotoxumab Was Not Better Than FMT Alone in Inflammatory Bowel Disease Patients and Recurrent Clostridioides difficile Infection: Results from a Randomized Controlled Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Jessica R. Allegretti, MD, MPH, FACG1, Jordan Axelrad, MD, MPH2, Rahul S. Dalal, MD, MPH3, Heidy Cabral, BS, MS3, Alexander Carlin, BS3, Jenna Marcus, BA4, Colleen R. Kelly, MD, FACG4, Ari Grinspan, MD5, Monika Fischer, MD, MS6
1Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 2NYU Grossman School of Medicine, New York, NY; 3Brigham and Women's Hospital, Boston, MA; 4Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6Indiana University, Indianapolis, IN
Introduction: Fecal microbiota transplantation (FMT) has been shown to be safe and effective among inflammatory bowel disease (IBD) patients with recurrent Clostridioides difficile infection (rCDI). Additionally, bezlotoxumab, a fully human monoclonal antibody that binds to C. difficile toxin B, is indicated to prevent recurrence of CDI in adults at risk for rCDI. We aimed to assess the impact of FMT in combination with bezlotoxumab in patients with IBD and rCDI.
Methods: Multicenter randomized placebo-controlled trial. Patients with IBD and 2 or more episodes of CDI received a colonoscopic FMT. Patients were randomized 1:1 to receive a single bezlotoxumab infusion or placebo prior to the FMT. CDI testing (GDH/EIA toxin and PCR) was performed pre-FMT, and at 1, 8, and 12 weeks post-FMT. The primary outcome was CDI recurrence up to week 8 defined as diarrhea and EIA+ toxin test. The secondary outcome assessed was CDI decolonization post-treatment through week 12 defined as negative PCR testing through week 12. Risk factors for ongoing colonization at week 12 were assessed using univariate logistic regression.
Results: 61 participants were enrolled with a median age of 38 (IQR 28,54), 54% were male (n=33), 20 of which had Crohn’s disease (CD) (mean HBI =7.2) and 41 had ulcerative colitis (UC) (mean Partial Mayo Score =3.2). Median baseline fecal calprotectin was 635 (IQR 150, 2615). Thirty patients were in the treatment arm and 31 in the placebo arm, with no significant differences between groups. 5 participants (8%) experienced a CDI recurrence with confirmed EIA+ stool, of these 4 were in the treatment arm and 1 was in the placebo arm (13% vs 3%, p=0.15). With regards to C.difficile colonization, though not statistically significant, more patients in the treatment arm were decolonized compared to placebo at week 1 (82% vs 68%, p=0.22) and at week 12 (83% vs 72%, p=0.34). Risk factors for ongoing colonization were assessed (Table 1). Steroid use at the time of FMT led to a significant increased risk of ongoing colonization of C.difficile at week 12 post FMT (OR: 4.90, 95% CI: 1.18-20.37, p=0.03).
Discussion: This is the first clinical trial to assess the effect of FMT in combination with bezlotoxumab in patients with IBD and rCDI. The data suggests no clear efficacy benefit to a combination approach. Steroid use at the time of FMT was a significant risk factor for ongoing colonization 12 weeks post FMT, a critical factor for future risk of recurrence.

Figure: Table 1: Risk Factors for Ongoing C.difficile Colonization post FMT
Disclosures:
Jessica R. Allegretti: Abbvie – Consultant, Speakers Bureau. Artugen – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant. Finch Therapeutics – Consultant. Janssen – Consultant. Merck – Grant/Research Support. Pfizer – Consultant. Seres – Consultant.
Jordan Axelrad: Abbvie – Consultant. Adiso – Consultant. Biomerieux – Consultant. BMS – Consultant. Fresenius – Consultant. Genentech – Grant/Research Support. Janssen – Consultant. Pfizer – Consultant.
Rahul Dalal: Centaur Labs – Consultant. Janssen – Consultant, Grant/Research Support. Pfizer – Grant/Research Support. Takeda – Consultant.
Heidy Cabral indicated no relevant financial relationships.
Alexander Carlin indicated no relevant financial relationships.
Jenna Marcus indicated no relevant financial relationships.
Colleen Kelly indicated no relevant financial relationships.
Ari Grinspan indicated no relevant financial relationships.
Monika Fischer: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Eli Lilly and Company – Consultant. Ferring Pharmaceuticals – Consultant. Janssen – Consultant. Pfizer – Consultant. Rebiotix – Consultant. Scioto Biosciences – Consultant. Seres Therapeutics – Consultant.
Jessica R. Allegretti, MD, MPH, FACG1, Jordan Axelrad, MD, MPH2, Rahul S. Dalal, MD, MPH3, Heidy Cabral, BS, MS3, Alexander Carlin, BS3, Jenna Marcus, BA4, Colleen R. Kelly, MD, FACG4, Ari Grinspan, MD5, Monika Fischer, MD, MS6, 65, Fecal Microbiota Transplantation (FMT) With Bezlotoxumab Was Not Better Than FMT Alone in Inflammatory Bowel Disease Patients and Recurrent Clostridioides difficile Infection: Results from a Randomized Controlled Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.



