Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4B - IBD
69 - Real World Comparison of Effectiveness, Durability, and Safety of First-Line Advanced Therapies for Ulcerative Proctitis
Wednesday, October 30, 2024
9:10 AM - 9:20 AM ET
Location: Terrace Ballroom 2-3

Rahul Dalal, MD, MPH
Brigham and Women's Hospital
Boston, MA
Presenting Author(s)
Rahul Dalal, MD, MPH1, Lindsay Clarke, MD2, Alexander Carlin, BS1, Heidy Cabral, BS, MS1, Jessica R. Allegretti, MD, MPH, FACG3
1Brigham and Women's Hospital, Boston, MA; 2Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 3Brigham and Women’s Hospital, Harvard Medical School, Boston, MA
Introduction: Patients with ulcerative proctitis (UP) experience significant symptomatology but are often excluded from clinical trials of advanced therapies (ATs). We compared real-world outcomes of ATs in bio-naïve patients with UP.
Methods: In this retrospective cohort study, adults initiated ATs for UP between 1/1/14-6/1/23 at a large academic health system. UP diagnoses were confirmed via review of endoscopic reports documenting inflammation < 15 cm from the anal verge. The primary outcome was steroid-free clinical remission (SFCR) at 52 +/- 4 weeks (clinical colitis activity index [SCCAI] <2 or provider global assessment when SCCAI was not documented). The secondary outcome was treatment persistence at 52 weeks. Additional outcomes included SFCR at 12 +/- 4 weeks, endoscopic response (reduction in Mayo endoscopic subscore [MES] by >1 pt), and adverse events (AEs). Multivariable logistic regression was used to compare SFCR at 52 weeks for vedolizumab (vedo) vs anti-TNFs (both comprised >90% of cohort). Kaplan-Meier and multivariable Cox regression analyses were used to compare treatment persistence for vedo vs anti-TNFs. Multivariable analyses adjusted for age, sex, MES, and oral steroid use.
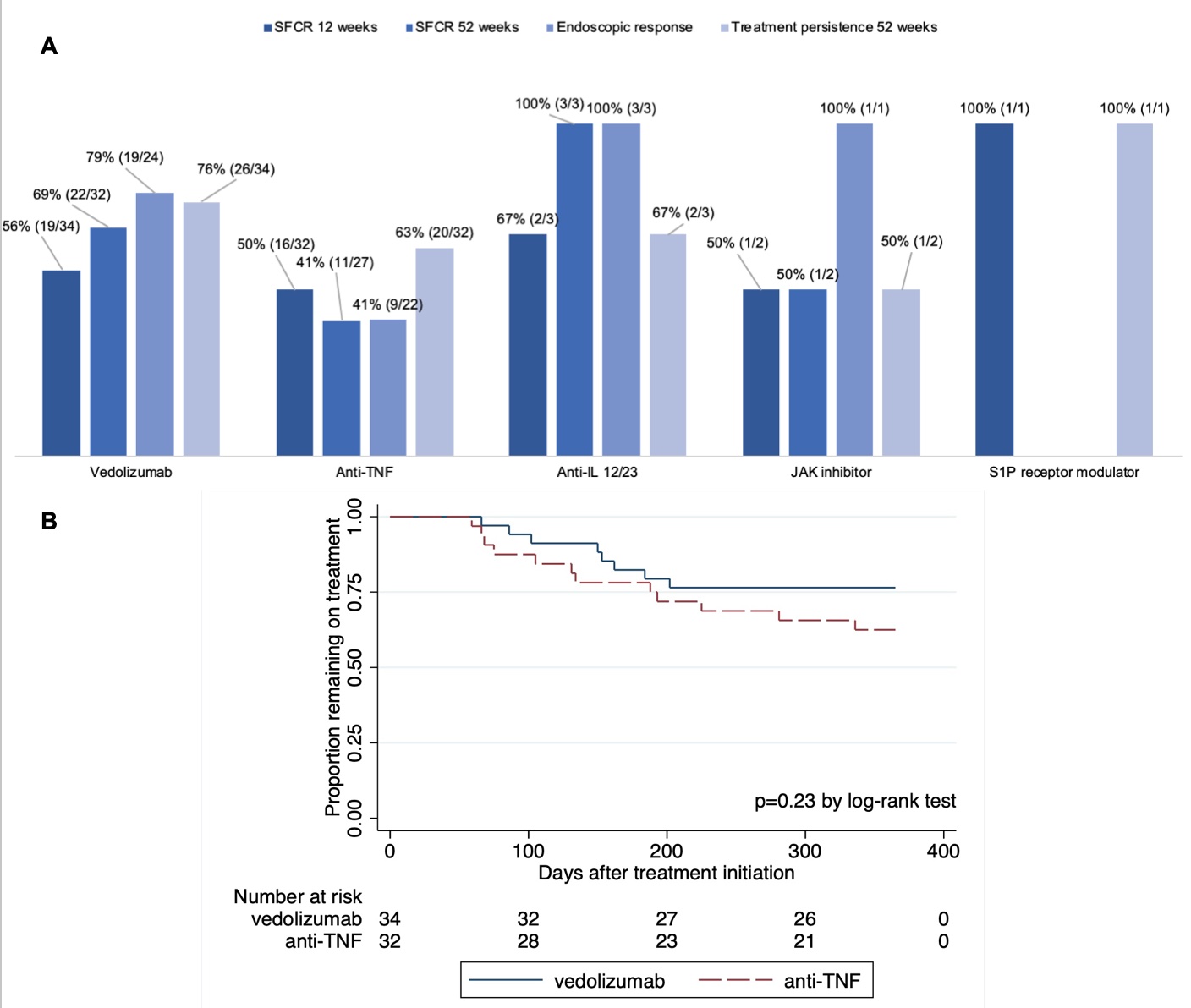
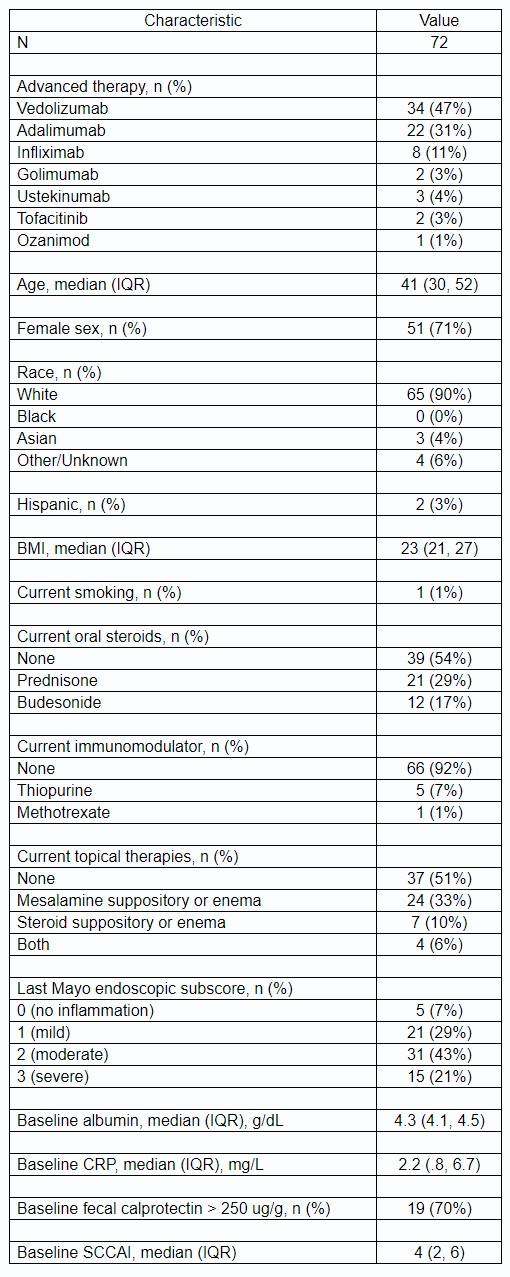
Results: 72 adults initiated vedo (47%), anti-TNFs (44%, of which 69% was adalimumab), ustekinumab (4%), tofacitinib (3%), and ozanimod (1%) for UP. Baseline characteristics and unadjusted outcomes are presented in Table 1 and Fig 1A. After multivariable logistic regression, vedo was associated with significantly higher adjusted odds of SFCR at 52 weeks vs anti-TNFs (OR 3.8, p=0.03). Kaplan-Meier analysis (Fig 1B) and multivariable Cox regression showed lower treatment persistence and a higher hazard of discontinuation for anti-TNFs vs vedo, which were non-significant (HR 1.8, p=0.20). In a subgroup analysis, adalimumab was associated with a significantly higher hazard of discontinuation vs vedo (HR 2.8, p=0.04). AEs were consistent with known safety profiles: n=8 vedo (4 arthralgia, 1 fatigue, 1 lyme disease, 1 rash, 1 spontaneous abortion), n=11 anti-TNFs (1 interstitial lung disease, 1 fatigue, 1 hypersensitivity reaction, 3 injection site reactions, 1 migraines, 1 myalgias, 3 rash), n=1 ustekinumab (nausea/vomiting).
Discussion: Vedo may be associated with higher odds of SFCR and greater durability for UP vs anti-TNFs (primarily adalimumab). Larger cohorts are needed to understand the comparative effectiveness of various of ATs for this poorly studied subtype of UC.
Disclosures:
Rahul Dalal, MD, MPH1, Lindsay Clarke, MD2, Alexander Carlin, BS1, Heidy Cabral, BS, MS1, Jessica R. Allegretti, MD, MPH, FACG3, 69, Real World Comparison of Effectiveness, Durability, and Safety of First-Line Advanced Therapies for Ulcerative Proctitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Brigham and Women's Hospital, Boston, MA; 2Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 3Brigham and Women’s Hospital, Harvard Medical School, Boston, MA
Introduction: Patients with ulcerative proctitis (UP) experience significant symptomatology but are often excluded from clinical trials of advanced therapies (ATs). We compared real-world outcomes of ATs in bio-naïve patients with UP.
Methods: In this retrospective cohort study, adults initiated ATs for UP between 1/1/14-6/1/23 at a large academic health system. UP diagnoses were confirmed via review of endoscopic reports documenting inflammation < 15 cm from the anal verge. The primary outcome was steroid-free clinical remission (SFCR) at 52 +/- 4 weeks (clinical colitis activity index [SCCAI] <2 or provider global assessment when SCCAI was not documented). The secondary outcome was treatment persistence at 52 weeks. Additional outcomes included SFCR at 12 +/- 4 weeks, endoscopic response (reduction in Mayo endoscopic subscore [MES] by >1 pt), and adverse events (AEs). Multivariable logistic regression was used to compare SFCR at 52 weeks for vedolizumab (vedo) vs anti-TNFs (both comprised >90% of cohort). Kaplan-Meier and multivariable Cox regression analyses were used to compare treatment persistence for vedo vs anti-TNFs. Multivariable analyses adjusted for age, sex, MES, and oral steroid use.
Results: 72 adults initiated vedo (47%), anti-TNFs (44%, of which 69% was adalimumab), ustekinumab (4%), tofacitinib (3%), and ozanimod (1%) for UP. Baseline characteristics and unadjusted outcomes are presented in Table 1 and Fig 1A. After multivariable logistic regression, vedo was associated with significantly higher adjusted odds of SFCR at 52 weeks vs anti-TNFs (OR 3.8, p=0.03). Kaplan-Meier analysis (Fig 1B) and multivariable Cox regression showed lower treatment persistence and a higher hazard of discontinuation for anti-TNFs vs vedo, which were non-significant (HR 1.8, p=0.20). In a subgroup analysis, adalimumab was associated with a significantly higher hazard of discontinuation vs vedo (HR 2.8, p=0.04). AEs were consistent with known safety profiles: n=8 vedo (4 arthralgia, 1 fatigue, 1 lyme disease, 1 rash, 1 spontaneous abortion), n=11 anti-TNFs (1 interstitial lung disease, 1 fatigue, 1 hypersensitivity reaction, 3 injection site reactions, 1 migraines, 1 myalgias, 3 rash), n=1 ustekinumab (nausea/vomiting).
Discussion: Vedo may be associated with higher odds of SFCR and greater durability for UP vs anti-TNFs (primarily adalimumab). Larger cohorts are needed to understand the comparative effectiveness of various of ATs for this poorly studied subtype of UC.

Figure: Figure 1. A. Unadjusted outcomes by therapy class. B. Kaplan-Meier analysis.
Abbreviations: SFCR = steroid-free clinical remission
Abbreviations: SFCR = steroid-free clinical remission

Table: Table 1. Baseline Characteristics.
Abbreviations: IQR = interquartile range, BMI = body mass index, CRP = C-reactive protein, SCCAI = simple clinical colitis activity index
Abbreviations: IQR = interquartile range, BMI = body mass index, CRP = C-reactive protein, SCCAI = simple clinical colitis activity index
Disclosures:
Rahul Dalal: Centaur Labs – Consultant. Janssen – Consultant, Grant/Research Support. Pfizer – Grant/Research Support. Takeda – Consultant.
Lindsay Clarke indicated no relevant financial relationships.
Alexander Carlin indicated no relevant financial relationships.
Heidy Cabral indicated no relevant financial relationships.
Jessica R. Allegretti: Abbvie – Consultant, Speakers Bureau. Artugen – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant. Finch Therapeutics – Consultant. Janssen – Consultant. Merck – Grant/Research Support. Pfizer – Consultant. Seres – Consultant.
Rahul Dalal, MD, MPH1, Lindsay Clarke, MD2, Alexander Carlin, BS1, Heidy Cabral, BS, MS1, Jessica R. Allegretti, MD, MPH, FACG3, 69, Real World Comparison of Effectiveness, Durability, and Safety of First-Line Advanced Therapies for Ulcerative Proctitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


