Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4B - IBD
70 - Efficacy and Safety of Upadacitinib Maintenance Treatment in Patients With Moderately to Severely Active Crohn’s Disease: Two Year Results From the U-ENDURE Long-Term Extension Study
Wednesday, October 30, 2024
9:20 AM - 9:30 AM ET
Location: Terrace Ballroom 2-3

Edward V. Loftus, Jr., MD, FACG
Maxine and Jack Zarrow Family Professor of Gastroenterology
Mayo Clinic College of Medicine and Science
Rochester, MN
Presenting Author(s)
Geert R. D'Haens, MD, PhD1, Edouard Louis, MD2, Edward V. Loftus, MD, FACG3, Miguel D Regueiro, MD4, Vipul Jairath, MBChB5, Fernando Magro, 6, Hiroshi Nakase, MD7, Elena Dubcenco, MD8, Ana Paula Lacerda, MD8, Tian Feng, MD8, Benjamin Duncan, PhD8, Tao Wang, MD8, Samuel Anyanwu, MD8, Fernando Aponte, MD8, Irina Blumenstein, MD, PhD9
1Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands; 2University Hospital CHU of Liège, Liege, Liege, Belgium; 3Mayo Clinic College of Medicine and Science, Rochester, MN; 4Cleveland Clinic, Cleveland, OH; 5Western University, London, ON, Canada; 6Centro Hospitalar São João, Porto, Porto, Portugal; 7Sapporo Medical University School of Medicine, Sapporo, Hokkaido, Japan; 8AbbVie, North Chicago, IL; 9Goethe University Hospital, Frankfurt, Hamburg, Germany
Introduction: Upadacitinib (UPA) is an oral Janus kinase inhibitor approved for treating moderately to severely active Crohn’s disease (CD). We evaluated UPA maintenance therapy efficacy for up to 2 years (yrs) and safety outcomes for at least 2 yrs (up to 5 yrs) in the ongoing U-ENDURE long-term extension (LTE) substudy.
Methods: Patients who completed the U-ENDURE 52-week (wk) maintenance study were eligible for the subsequent LTE substudy and continued their previously assigned treatment (UPA 15 mg [UPA15] once daily [QD] or UPA 30 mg [UPA30] QD). Efficacy was assessed from LTE wk0 to wk48. Clinical remission (per stool frequency/abdominal pain score [SF/APS] and CD Activity Index [CDAI]), endoscopic response, endoscopic remission, were evaluated using both as observed (AO) and nonresponder imputation (NRI) methods; mean change from induction baseline (BL) in high-sensitivity C-reactive protein (hs-CRP) and fecal calprotectin (FCP) were evaluated using AO and modified as observed (mAO) methods. Safety was assessed from maintenance wk0, with up to 260 cumulative wks of maintenance and LTE treatment exposure (cutoff date: 19 Dec 2023).
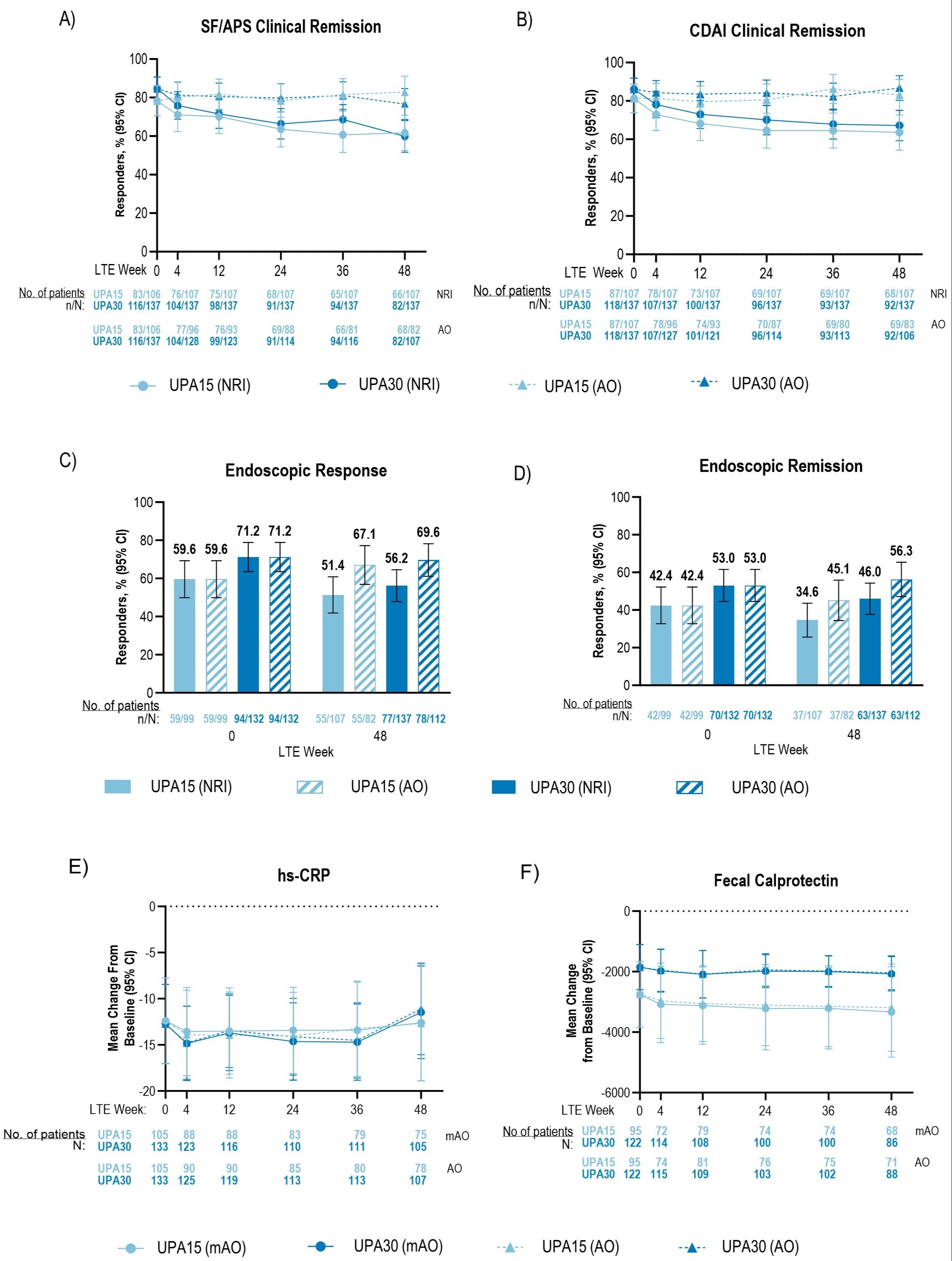
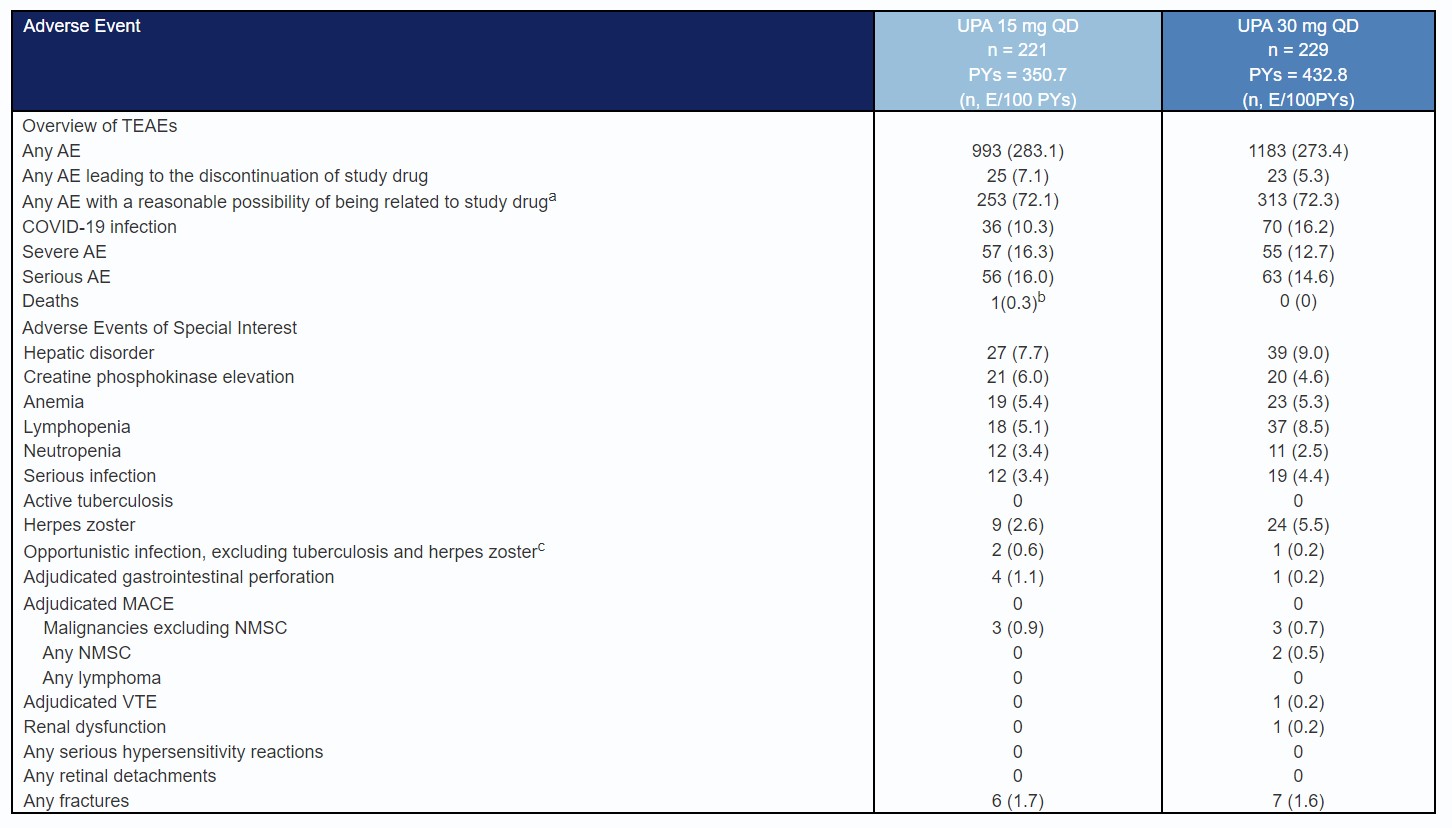
Results: From LTE wk0 to wk48, AO efficacy rates remained stable in each treatment group for SF/APS clinical remission (UPA15: 78.3% to 82.9%; UPA30: 84.7% to 76.6%; Fig.1A), CDAI clinical remission (UPA15: 81.3% to 83.1%; UPA30: 86.1% to 86.8%; Fig.1B), endoscopic response (UPA15: 59.6% to 67.1%; UPA30: 71.2% to 69.6%; Fig.1C), and endoscopic remission (UPA15: 42.4% to 45.1%; UPA30: 53.0% to 56.3%; Fig.1D). Clinical or endoscopic remission was sustained at LTE wk0 through wk48 as follows: clinical remission per SF/APS: UPA15 93.8%; UPA30 84.4%; per CDAI: UPA15 94.0%; UPA30 91.1%; endoscopic remission: UPA15 73.0%; UPA30 86.9% [AO]. Respectively, hs-CRP and FCP mean change from induction BL (UPA15 -12.4, -2752; UPA30 -12.7, -1850 [AO]) were stable through wk48 (UPA15 -12.7, -3191; UPA30 -11.1, -2038 [AO]) (Fig. 1E-F). Similar results were found per NRI (for binary variables) or mAO analysis (for continuous variables). Event rates for serious, severe, and most adverse events of special interest were similar among UPA-treated groups, except for numerically higher rates of COVID-19 infection, herpes zoster, and lymphopenia among UPA30 vs. UPA15-treated patients.
Discussion: Sustained efficacy for clinical, endoscopic, and inflammatory markers was observed in patients who completed up to 2 yrs of UPA maintenance therapy, with no new safety signals identified.
Disclosures:
Geert R. D'Haens, MD, PhD1, Edouard Louis, MD2, Edward V. Loftus, MD, FACG3, Miguel D Regueiro, MD4, Vipul Jairath, MBChB5, Fernando Magro, 6, Hiroshi Nakase, MD7, Elena Dubcenco, MD8, Ana Paula Lacerda, MD8, Tian Feng, MD8, Benjamin Duncan, PhD8, Tao Wang, MD8, Samuel Anyanwu, MD8, Fernando Aponte, MD8, Irina Blumenstein, MD, PhD9, 70, Efficacy and Safety of Upadacitinib Maintenance Treatment in Patients With Moderately to Severely Active Crohn’s Disease: Two Year Results From the U-ENDURE Long-Term Extension Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands; 2University Hospital CHU of Liège, Liege, Liege, Belgium; 3Mayo Clinic College of Medicine and Science, Rochester, MN; 4Cleveland Clinic, Cleveland, OH; 5Western University, London, ON, Canada; 6Centro Hospitalar São João, Porto, Porto, Portugal; 7Sapporo Medical University School of Medicine, Sapporo, Hokkaido, Japan; 8AbbVie, North Chicago, IL; 9Goethe University Hospital, Frankfurt, Hamburg, Germany
Introduction: Upadacitinib (UPA) is an oral Janus kinase inhibitor approved for treating moderately to severely active Crohn’s disease (CD). We evaluated UPA maintenance therapy efficacy for up to 2 years (yrs) and safety outcomes for at least 2 yrs (up to 5 yrs) in the ongoing U-ENDURE long-term extension (LTE) substudy.
Methods: Patients who completed the U-ENDURE 52-week (wk) maintenance study were eligible for the subsequent LTE substudy and continued their previously assigned treatment (UPA 15 mg [UPA15] once daily [QD] or UPA 30 mg [UPA30] QD). Efficacy was assessed from LTE wk0 to wk48. Clinical remission (per stool frequency/abdominal pain score [SF/APS] and CD Activity Index [CDAI]), endoscopic response, endoscopic remission, were evaluated using both as observed (AO) and nonresponder imputation (NRI) methods; mean change from induction baseline (BL) in high-sensitivity C-reactive protein (hs-CRP) and fecal calprotectin (FCP) were evaluated using AO and modified as observed (mAO) methods. Safety was assessed from maintenance wk0, with up to 260 cumulative wks of maintenance and LTE treatment exposure (cutoff date: 19 Dec 2023).
Results: From LTE wk0 to wk48, AO efficacy rates remained stable in each treatment group for SF/APS clinical remission (UPA15: 78.3% to 82.9%; UPA30: 84.7% to 76.6%; Fig.1A), CDAI clinical remission (UPA15: 81.3% to 83.1%; UPA30: 86.1% to 86.8%; Fig.1B), endoscopic response (UPA15: 59.6% to 67.1%; UPA30: 71.2% to 69.6%; Fig.1C), and endoscopic remission (UPA15: 42.4% to 45.1%; UPA30: 53.0% to 56.3%; Fig.1D). Clinical or endoscopic remission was sustained at LTE wk0 through wk48 as follows: clinical remission per SF/APS: UPA15 93.8%; UPA30 84.4%; per CDAI: UPA15 94.0%; UPA30 91.1%; endoscopic remission: UPA15 73.0%; UPA30 86.9% [AO]. Respectively, hs-CRP and FCP mean change from induction BL (UPA15 -12.4, -2752; UPA30 -12.7, -1850 [AO]) were stable through wk48 (UPA15 -12.7, -3191; UPA30 -11.1, -2038 [AO]) (Fig. 1E-F). Similar results were found per NRI (for binary variables) or mAO analysis (for continuous variables). Event rates for serious, severe, and most adverse events of special interest were similar among UPA-treated groups, except for numerically higher rates of COVID-19 infection, herpes zoster, and lymphopenia among UPA30 vs. UPA15-treated patients.
Discussion: Sustained efficacy for clinical, endoscopic, and inflammatory markers was observed in patients who completed up to 2 yrs of UPA maintenance therapy, with no new safety signals identified.

Figure: Figure 1. Clinical, Endoscopic, and Inflammatory Markers in Patients Treated With Upadacitinib Through Week 48 of the U-ENDURE Long-Term Extension Study. Patients were blinded until the last patient completed maintenance wk 52. AO analysis used all available data up to the initiation of open-label UPA rescue and did not impute values for missing data. For NRI analysis on binary variables, patients were categorized as “nonresponder” after initiation of any protocol rescue medications or missing data. For mAO analysis on continuous variables, all available data up to the initiation of any protocol rescue medications were used and did not impute values for missing data. 95% CIs for the response rate were based on the normal approximation to the binomial distribution.
A) SF/APS Clinical remission was defined as average daily very soft or liquid SF ≤ 2.8 and average daily AP score ≤ 1.0 and both not greater than induction BL.
B) Clinical remission per CDAI was defined as CDAI < 150.
C) Endoscopic response was defined as a decrease in SES-CD > 50% from BL of the induction study (or for patients with an SES-CD of 4 at baseline of the induction study, at least a 2-point reduction from baseline), as scored by a central reviewer. Endoscopies were performed annually.
D) Endoscopic remission was defined as SES-CD ≤ 4 and at least a 2-point reduction from BL and no subscore > 1 in any individual variable, as scored by a central reviewer and measured up to wk 48.
For assessment of (E) hs-CRP and (F) FCP, BL was defined as wk 0 of induction for both induction and maintenance.
AO, as observed; BL, baseline; CDAI, Crohn’s disease activity index; CI, confidence intervals; FCP, fecal calprotectin; hs-CRP, high-sensitivity C-reactive protein; LTE, long-term extension; NRI, nonresponder imputation; mAO, modified as observed; SES-CD, Simplified Endoscopic Score for Patients with Crohn’s Disease; SF/APS, stool frequency/abdominal pain score; wk, week.
A) SF/APS Clinical remission was defined as average daily very soft or liquid SF ≤ 2.8 and average daily AP score ≤ 1.0 and both not greater than induction BL.
B) Clinical remission per CDAI was defined as CDAI < 150.
C) Endoscopic response was defined as a decrease in SES-CD > 50% from BL of the induction study (or for patients with an SES-CD of 4 at baseline of the induction study, at least a 2-point reduction from baseline), as scored by a central reviewer. Endoscopies were performed annually.
D) Endoscopic remission was defined as SES-CD ≤ 4 and at least a 2-point reduction from BL and no subscore > 1 in any individual variable, as scored by a central reviewer and measured up to wk 48.
For assessment of (E) hs-CRP and (F) FCP, BL was defined as wk 0 of induction for both induction and maintenance.
AO, as observed; BL, baseline; CDAI, Crohn’s disease activity index; CI, confidence intervals; FCP, fecal calprotectin; hs-CRP, high-sensitivity C-reactive protein; LTE, long-term extension; NRI, nonresponder imputation; mAO, modified as observed; SES-CD, Simplified Endoscopic Score for Patients with Crohn’s Disease; SF/APS, stool frequency/abdominal pain score; wk, week.

Table: Table 1. Overview of Treatment-Emergent Adverse Events in Patients Treated With Upadacitinib From Week 0 of the U-ENDURE Maintenance Study, With Up to 260 Cumulative Weeks of Maintenance and Long-Term Extension Exposure. Exposure-adjusted event rate is expressed as number of events per 100 patient-years (E/100 PYs). TEAEs are defined as any AEs with an onset date on or after the first dose of the study drug in the maintenance period and up to 30 days past the last dose of the study drug in the maintenance or LTE period or until one day prior to the rescue in the maintenance or LTE period.
aAs assessed by the study investigator
bAn event of suicide with no reasonable possibility of being related to the study drug as assessed by the investigator.
cThree events of opportunistic infections (excluding tuberculosis and herpes zoster) were reported: esophageal candidiasis and Pneumocystis jirovecii pneumonia in the UPA 15 mg group, and esophageal candidiasis in the UPA 30 mg group. The event of Pneumocystis jirovecii pneumonia was serious and led to the discontinuation of study drug.
dOne patient on UPA 15 mg had an event of gastrointestinal perforation that appeared twice in the table (diverticular perforation and abdominal abscess).
AE, adverse event; COVID-19, coronavirus 2019; E, event; LTE, long-term extension; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PBO, placebo; PY, patient-year; TEAE, treatment-emergent adverse event; UPA, upadacitinib; VTE, Venous thromboembolic events.
aAs assessed by the study investigator
bAn event of suicide with no reasonable possibility of being related to the study drug as assessed by the investigator.
cThree events of opportunistic infections (excluding tuberculosis and herpes zoster) were reported: esophageal candidiasis and Pneumocystis jirovecii pneumonia in the UPA 15 mg group, and esophageal candidiasis in the UPA 30 mg group. The event of Pneumocystis jirovecii pneumonia was serious and led to the discontinuation of study drug.
dOne patient on UPA 15 mg had an event of gastrointestinal perforation that appeared twice in the table (diverticular perforation and abdominal abscess).
AE, adverse event; COVID-19, coronavirus 2019; E, event; LTE, long-term extension; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PBO, placebo; PY, patient-year; TEAE, treatment-emergent adverse event; UPA, upadacitinib; VTE, Venous thromboembolic events.
Disclosures:
Geert D'Haens: AbbVie – Advisor or Review Panel Member, Speakers Bureau. Agomab Therapeutics – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. Allergan – Advisor or Review Panel Member. Alphabiomics – Advisor or Review Panel Member. AstraZeneca – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Speakers Bureau. Ferring – Advisor or Review Panel Member. Galapagos – Advisor or Review Panel Member, Speakers Bureau. GlaxoSmithKline – Advisor or Review Panel Member. Immunic – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Seres – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau. Tillotts – Advisor or Review Panel Member, Speakers Bureau. Ventyx – Advisor or Review Panel Member.
Edouard Louis: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Arena – Speakers Bureau. Celgene – Advisory Committee/Board Member. Falk – Speakers Bureau. Ferring – Advisory Committee/Board Member, Speakers Bureau. Galapagos – Advisory Committee/Board Member. Gilead – Advisory Committee/Board Member. Hospira – Advisory Committee/Board Member, Speakers Bureau. Janseen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. MSD – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Takeda – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau.
Edward Loftus: AbbVie – Consultant, Grant/Research Support. Amgen – Consultant. Astellas – Consultant. AstraZeneca – Grant/Research Support. Avalo – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Exact Sciences – Stock-publicly held company(excluding mutual/index funds). Fresenius Kabi – Consultant. Genentech – Consultant, Grant/Research Support. Gilead – Consultant, Grant/Research Support. Iota Biosciences – Consultant. Iterative Health – Grant/Research Support. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Morphic Therapeutics – Consultant. Ono Pharma – Consultant. Receptos – Grant/Research Support. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support. TR1X Bio – Consultant. UCB – Consultant, Grant/Research Support.
Miguel D Regueiro: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Employee, Grant/Research Support, Speakers Bureau. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Advisory Committee/Board Member, Consultant. Enthera – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Advisory Committee/Board Member, Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Advisory Committee/Board Member, Consultant. JAMP – Advisory Committee/Board Member, Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. London Health Sciences Centre – Employee. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Advisory Committee/Board Member, Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. SCOPE – Advisory Committee/Board Member, Consultant. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Synedgen – Advisory Committee/Board Member, Consultant. Takeda – Consultant, Grant/Research Support, Speakers Bureau. TD Securities – Advisory Committee/Board Member, Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Fernando Magro: AbbVie – Received honoraria, Speakers Bureau. Biogen – Received honoraria, Speakers Bureau. Dr. Falk Pharma – Received honoraria. Ferring Pharmaceuticals – Received honoraria, Speakers Bureau. Hospira – Received honoraria, Speakers Bureau. Laboratórios Vitória – Received honoraria, Speakers Bureau. Merck Sharp & Dohme – Received honoraria, Speakers Bureau. Vifor Pharma – Received honoraria, Speakers Bureau.
Hiroshi Nakase: AbbVie – Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Celgene – Grant/Research Support. Daiichi Sankyo Company – Grant/Research Support. EA Pharma – Grant/Research Support. Hoya Group Pentax Medical – Grant/Research Support. Janssen – Grant/Research Support. JIMRO – Grant/Research Support. Kissei Pharmaceutical – Grant/Research Support. Kyorin Pharmaceutical – Grant/Research Support. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support. Mochida Pharmaceutical – Grant/Research Support. Nippon Kayaku – Grant/Research Support. Pfizer – Grant/Research Support. Takeda – Grant/Research Support. Zeria – Grant/Research Support.
Elena Dubcenco: AbbVie – Employee, Stock Options.
Ana Paula Lacerda: AbbVie – Employee, Stock Options.
Tian Feng: AbbVie – Employee, Stock Options.
Benjamin Duncan: AbbVie – Employee, Stock Options.
Tao Wang: AbbVie – Employee, Stock Options.
Samuel Anyanwu: AbbVie – Employee, Stock Options.
Fernando Aponte: AbbVie – Employee, Stock Options.
Irina Blumenstein: AbbVie – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. Arena – Consultant, Speakers Bureau. Biogen – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celgene – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Mylan – Speakers Bureau. Pfizer – Consultant, Stock Options. Shire – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau.
Geert R. D'Haens, MD, PhD1, Edouard Louis, MD2, Edward V. Loftus, MD, FACG3, Miguel D Regueiro, MD4, Vipul Jairath, MBChB5, Fernando Magro, 6, Hiroshi Nakase, MD7, Elena Dubcenco, MD8, Ana Paula Lacerda, MD8, Tian Feng, MD8, Benjamin Duncan, PhD8, Tao Wang, MD8, Samuel Anyanwu, MD8, Fernando Aponte, MD8, Irina Blumenstein, MD, PhD9, 70, Efficacy and Safety of Upadacitinib Maintenance Treatment in Patients With Moderately to Severely Active Crohn’s Disease: Two Year Results From the U-ENDURE Long-Term Extension Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


