Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4B - IBD
71 - Efficacy of Mirikizumab by Baseline Disease Location in Moderately to Severely Active Crohn’s Disease: Results of the Phase 3 VIVID-1 Study
Wednesday, October 30, 2024
9:30 AM - 9:40 AM ET
Location: Terrace Ballroom 2-3

Edward Barnes, MD, MPH, FACG
University of North Carolina at Chapel Hill School of Medicine
Chapel Hill, NC
Presenting Author(s)
Award: ACG Governors Award for Excellence in Clinical Research
Edward Barnes, MD, MPH, FACG1, Peter Bossuyt, 2, Marc Ferrante, MD, PhD3, Britta Siegmund, MD4, Frederick Durand, 5, Deborah A. Fisher, MD, MHS6, Nathan Morris, 5, Guanglei Yu, 5, Sonja M.. Bragg, BS, MPH5, Aline Charabaty, MD7
1University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC; 2Imelda GI Clinical Research Center, Imelda General Hospital, Bonheiden, Antwerpen, Belgium; 3University Hospitals, Leuven, Vlaams-Brabant, Belgium; 4Med. Klinik für Gastroenterologie, Infektiologie, Rheumatologie, Charité – Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, Campus Benjamin Franklin, Hindenburgdamm, Berlin, Germany; 5Eli Lilly and Company, Indianapolis, IN; 6Eli Lilly and Company, Raleigh, NC; 7Johns Hopkins University School of Medicine, Washington, DC
Introduction: Results from the phase 3 VIVID-1 study (NCT03926130) showed the efficacy of mirikizumab (miri), an anti-IL-23p19 monoclonal antibody, versus (vs) placebo (PBO), and ustekinumab (uste) in moderately-to-severely active Crohn’s disease (CD)1. Here, we present miri efficacy vs PBO across ileal, colonic, and ileocolonic baseline (BL) disease locations assessed by endoscopy in VIVID-1.
Methods: Patients were randomized 6:3:2 to miri (N=579), uste (N=287), or PBO (N=199). Miri was administered at week (W) 0, W4 and W8 (induction), then every 4 weeks from W12 to W52 (maintenance). At W12, PBO responders continued PBO to W52. PBO non-responders received blinded miri as described above. Endpoints in this subgroup analysis, based on the primary analysis set population, included the co-primary composite endpoints (A) clinical remission-composite: Patient Reported Outcome (PRO) clinical response at W12 and Crohn’s Disease Activity Index (CDAI) clinical remission at W52, and (B) endoscopic response-composite: PRO clinical response at W12 and endoscopic response at W52. Other endpoints were: 1) CDAI clinical remission at W12, 2) endoscopic response at W12, and 3) endoscopic remission-composite: PRO clinical response at W12 and endoscopic remission at W52. Non-responder imputation was used.
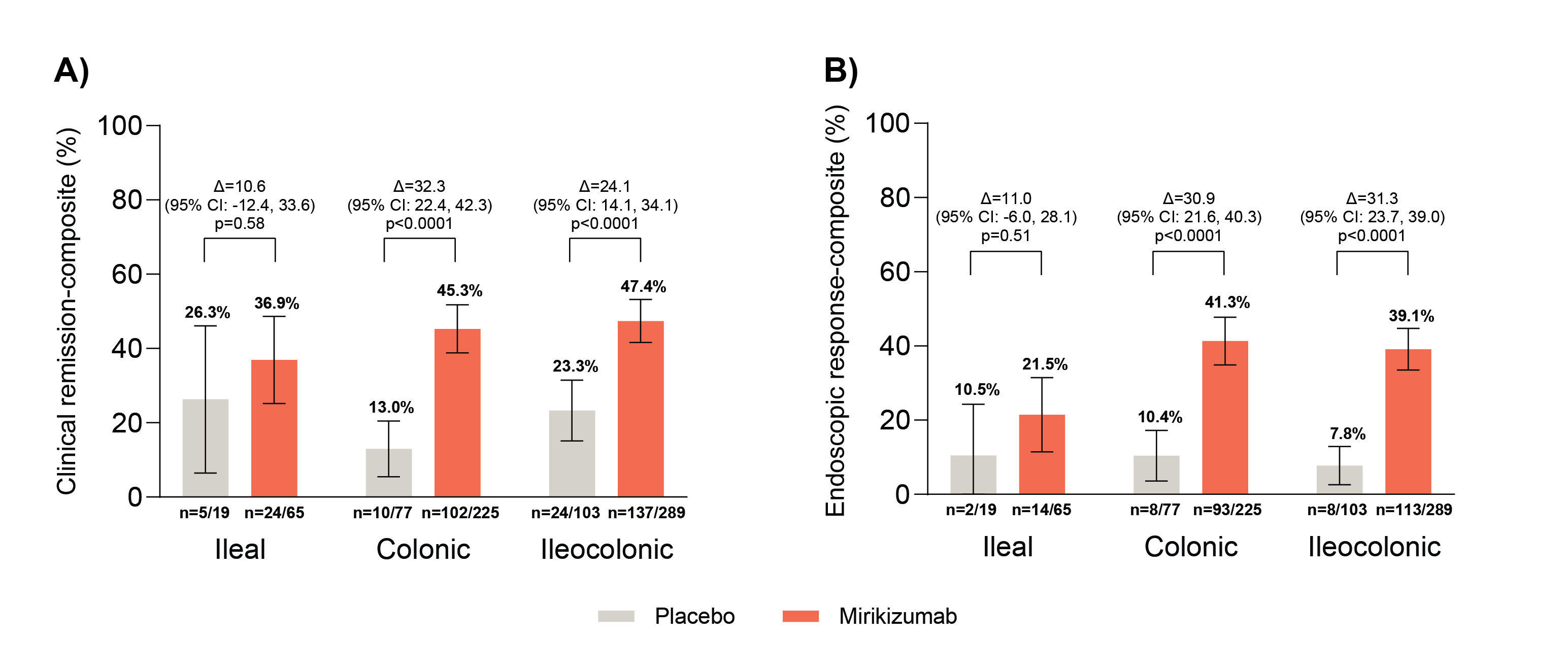
Results: Of miri and PBO patients, 11.2% (n=65) and 9.5% (n=19) had ileal, 38.9% (n=225) and 38.7% (n=77) had colonic, and 49.9% (n=289) and 51.8% (n=103) had ileocolonic BL CD location.
Figure shows miri met both co-primary endpoints vs PBO in the colonic and ileocolonic subgroups (all nominal p< 0.0001). Greater proportions of miri-treated patients vs PBO in the colonic and ileocolonic subgroups achieved W12 CDAI clinical remission, W12 endoscopic response, and endoscopic remission-composite (all nominally statistically significant). For ileal CD, miri response rates were numerically greater vs PBO for all reported endpoints except W12 CDAI clinical remission.
Discussion: These results by BL CD location are consistent with the VIVID-1 primary results, which demonstrated effectiveness of miri in achieving and maintaining symptomatic and endoscopic improvements up to 1 year. Analyses of patients with ileal-only disease were limited by the small sample size.
1Jairath et al. OP35 Efficacy of mirikizumab in comparison to ustekinumab in patients with moderate to severe Crohn’s disease: Results from the phase 3 VIVID 1 study. J Crohns Colitis 2024: 18Supplement_1, i62–i64.
Disclosures:
Edward Barnes, MD, MPH, FACG1, Peter Bossuyt, 2, Marc Ferrante, MD, PhD3, Britta Siegmund, MD4, Frederick Durand, 5, Deborah A. Fisher, MD, MHS6, Nathan Morris, 5, Guanglei Yu, 5, Sonja M.. Bragg, BS, MPH5, Aline Charabaty, MD7, 71, Efficacy of Mirikizumab by Baseline Disease Location in Moderately to Severely Active Crohn’s Disease: Results of the Phase 3 VIVID-1 Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Edward Barnes, MD, MPH, FACG1, Peter Bossuyt, 2, Marc Ferrante, MD, PhD3, Britta Siegmund, MD4, Frederick Durand, 5, Deborah A. Fisher, MD, MHS6, Nathan Morris, 5, Guanglei Yu, 5, Sonja M.. Bragg, BS, MPH5, Aline Charabaty, MD7
1University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC; 2Imelda GI Clinical Research Center, Imelda General Hospital, Bonheiden, Antwerpen, Belgium; 3University Hospitals, Leuven, Vlaams-Brabant, Belgium; 4Med. Klinik für Gastroenterologie, Infektiologie, Rheumatologie, Charité – Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, Campus Benjamin Franklin, Hindenburgdamm, Berlin, Germany; 5Eli Lilly and Company, Indianapolis, IN; 6Eli Lilly and Company, Raleigh, NC; 7Johns Hopkins University School of Medicine, Washington, DC
Introduction: Results from the phase 3 VIVID-1 study (NCT03926130) showed the efficacy of mirikizumab (miri), an anti-IL-23p19 monoclonal antibody, versus (vs) placebo (PBO), and ustekinumab (uste) in moderately-to-severely active Crohn’s disease (CD)1. Here, we present miri efficacy vs PBO across ileal, colonic, and ileocolonic baseline (BL) disease locations assessed by endoscopy in VIVID-1.
Methods: Patients were randomized 6:3:2 to miri (N=579), uste (N=287), or PBO (N=199). Miri was administered at week (W) 0, W4 and W8 (induction), then every 4 weeks from W12 to W52 (maintenance). At W12, PBO responders continued PBO to W52. PBO non-responders received blinded miri as described above. Endpoints in this subgroup analysis, based on the primary analysis set population, included the co-primary composite endpoints (A) clinical remission-composite: Patient Reported Outcome (PRO) clinical response at W12 and Crohn’s Disease Activity Index (CDAI) clinical remission at W52, and (B) endoscopic response-composite: PRO clinical response at W12 and endoscopic response at W52. Other endpoints were: 1) CDAI clinical remission at W12, 2) endoscopic response at W12, and 3) endoscopic remission-composite: PRO clinical response at W12 and endoscopic remission at W52. Non-responder imputation was used.
Results: Of miri and PBO patients, 11.2% (n=65) and 9.5% (n=19) had ileal, 38.9% (n=225) and 38.7% (n=77) had colonic, and 49.9% (n=289) and 51.8% (n=103) had ileocolonic BL CD location.
Figure shows miri met both co-primary endpoints vs PBO in the colonic and ileocolonic subgroups (all nominal p< 0.0001). Greater proportions of miri-treated patients vs PBO in the colonic and ileocolonic subgroups achieved W12 CDAI clinical remission, W12 endoscopic response, and endoscopic remission-composite (all nominally statistically significant). For ileal CD, miri response rates were numerically greater vs PBO for all reported endpoints except W12 CDAI clinical remission.
Discussion: These results by BL CD location are consistent with the VIVID-1 primary results, which demonstrated effectiveness of miri in achieving and maintaining symptomatic and endoscopic improvements up to 1 year. Analyses of patients with ileal-only disease were limited by the small sample size.
1Jairath et al. OP35 Efficacy of mirikizumab in comparison to ustekinumab in patients with moderate to severe Crohn’s disease: Results from the phase 3 VIVID 1 study. J Crohns Colitis 2024: 18Supplement_1, i62–i64.

Figure: Figure: Co-primary endpoints by baseline disease location.
Co-primary endpoints were: A) clinical remission-composite: PRO clinical response at W12 and CDAI clinical remission at W52, and B) endoscopic response-composite: PRO clinical response at W12 and endoscopic response at W52. Efficacy analyses, based on the primary analysis set population, included all randomized participants who had BL SES-CD≥ 7 (or ≥4 for isolated ileal disease) and received at least 1 dose of study intervention. Δ = difference versus placebo. The 95% confidence intervals are constructed using the asymptotic method, without continuity correction. P-value is from the Fisher’s exact test. Reported p-values are unadjusted for multiple testing.
Abbreviations: BL=baseline; CDAI=Crohn’s Disease Activity Index; CI=confidence interval; n=number of patients within the treatment arm that met the endpoints specified; PRO=Patient Reported Outcome; SES-CD=Simple Endoscopic Score for Crohn’s Disease; W=week.
Co-primary endpoints were: A) clinical remission-composite: PRO clinical response at W12 and CDAI clinical remission at W52, and B) endoscopic response-composite: PRO clinical response at W12 and endoscopic response at W52. Efficacy analyses, based on the primary analysis set population, included all randomized participants who had BL SES-CD≥ 7 (or ≥4 for isolated ileal disease) and received at least 1 dose of study intervention. Δ = difference versus placebo. The 95% confidence intervals are constructed using the asymptotic method, without continuity correction. P-value is from the Fisher’s exact test. Reported p-values are unadjusted for multiple testing.
Abbreviations: BL=baseline; CDAI=Crohn’s Disease Activity Index; CI=confidence interval; n=number of patients within the treatment arm that met the endpoints specified; PRO=Patient Reported Outcome; SES-CD=Simple Endoscopic Score for Crohn’s Disease; W=week.
Disclosures:
Edward Barnes: AbbVie, Inc. – Consultant. Boomerang – Consultant. Bristol-Meyers Squibb – Consultant. Direct Biologics – Consultant. Eli Lilly and Company – Advisor or Review Panel Member. Pfizer – Consultant. Target RWE – Consultant.
Peter Bossuyt: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. Amgen – Grant/Research Support. Arena pharmaceuticals – Advisory Committee/Board Member. Benelux – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member, Lecture fees. CAG – Lecture fees. Celltrion – Advisory Committee/Board Member, Lecture fees. CiRC Biosciences – Advisory Committee/Board Member. Dr. Falk Pharma – Advisory Committee/Board Member. Eli Lilly and Company – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. EPGS – Lecture fees. Galapagos NV – Advisory Committee/Board Member, Lecture fees. Globalport – Lecture fees. Hospira – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. Materia Prima – Lecture fees. Merck – Advisory Committee/Board Member. Mundipharma – Advisory Committee/Board Member, Grant/Research Support. Mylan – Grant/Research Support. Pentax – Advisory Committee/Board Member, Lecture fees. Pfizer – Grant/Research Support, Advisory board fees. PSI CRO – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Scope – Lecture fees. Takeda – Advisory Committee/Board Member, Lecture fees. Tetrameros – Advisory Committee/Board Member. Viatris – Grant/Research Support.
Marc Ferrante: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Agomab – Consultant. Amgen – Grant/Research Support, Speakers Bureau. Biogen – Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Celgene – Consultant. Celltrion – Consultant. Dr Falk Pharma – Speakers Bureau. EG Pharmaceuticals – Grant/Research Support. Eli Lilly and Company – Consultant, Grant/Research Support. Ferring – Speakers Bureau. Janssen – Grant/Research Support. Janssen-Cilag – Consultant, Speakers Bureau. Lamepro – Speakers Bureau. Medtronic – Consultant. MRM Health – Consultant. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Regeneron – Consultant. Samsung Bioepis – Consultant. Sandoz – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. ThermoFisher – Consultant. Truvion Healthcare – Speakers Bureau. Viatris – Grant/Research Support, Speakers Bureau.
Britta Siegmund: AbbVie – Consultant, Speakers Bureau. Arena Pharmaceuticals – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. CED Service GmbH – Consultant, Speakers Bureau. Celgene – Consultant, Speakers Bureau. Dr. Falk Pharma – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos NV – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Novartis – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Prometheus Therapeutics – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau.
Frederick Durand: Eli Lilly and Company – Employee, Stock Options.
Deborah Fisher: Eli Lilly and Company – Employee, Stock Options.
Nathan Morris: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Sonja Bragg: Eli Lilly and Company – Employee.
Aline Charabaty: abbvie – Consultant. Eli Lilly and Company – Advisor or Review Panel Member, Consultant. janssen – Advisor or Review Panel Member, Consultant. pfizer – Consultant. takeda – Advisor or Review Panel Member.
Edward Barnes, MD, MPH, FACG1, Peter Bossuyt, 2, Marc Ferrante, MD, PhD3, Britta Siegmund, MD4, Frederick Durand, 5, Deborah A. Fisher, MD, MHS6, Nathan Morris, 5, Guanglei Yu, 5, Sonja M.. Bragg, BS, MPH5, Aline Charabaty, MD7, 71, Efficacy of Mirikizumab by Baseline Disease Location in Moderately to Severely Active Crohn’s Disease: Results of the Phase 3 VIVID-1 Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.



