Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4B - IBD
74 - TUSCANY-2: A Dose-Ranging Phase IIb Study Evaluating Efficacy and Safety of RO7790121, an Antibody Against Tumor Necrosis Factor-Like Ligand 1A (Anti-TL1A) in Adults With Moderately to Severely Active Ulcerative Colitis
Wednesday, October 30, 2024
10:00 AM - 10:10 AM ET
Location: Terrace Ballroom 2-3

Jessica R. Allegretti, MD, MPH, FACG
Director, Crohn's and Colitis Center, Brigham and Women's Hospital; Associate Professor of Medicine
Harvard Medical School
Cambridge, MA
Presenting Author(s)
Silvio Danese, MD, PhD1, Jessica R. Allegretti, MD, MPH, FACG2, Stefan Schreiber, MD3, Laurent Peyrin-Biroulet, MD, PhD4, Vipul Jairath, MBChB5, Geert R. D'Haens, MD, PhD6, Jarosław Kierkuś, MD, PhD7, Rupert W. Leong, MD, FRACP8, Andres Yarur, MD9, Jacqueline McBride, PhD10, Daniela Bojic, MD, PhD11, Karen Lasch, MD10, Courtney Schiffman, PhD10, Brian G.. Feagan, MD5
1Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 2Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 3University Hospital, Kiel, Schleswig-Holstein, Germany; 4INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 5Western University, London, ON, Canada; 6Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands; 7The Children's Memorial Health Institute, Warsaw, Mazowieckie, Poland; 8Concord Hospital and Macquarie University Hospital, Sydney, New South Wales, Australia; 9Cedars-Sinai Medical Center, Los Angeles, CA; 10Genentech, Inc., a member of the Roche Group, South San Francisco, CA; 11F. Hoffmann-La Roche Ltd., Basel, Basel-Stadt, Switzerland
Introduction: RO7790121 (previously PF-06480605, RVT-3101), an anti-TL1A antibody, was effective in a previous open-label phase IIa study for the treatment of active ulcerative colitis (UC). We report results from TUSCANY-2, a phase IIb, randomized, dose-ranging study evaluating efficacy, safety and changes in fecal calprotectin (FCP) levels in patients with moderately to severely active UC receiving subcutaneous (SC) RO7790121.
Methods: In this treat-through study, patients aged 18–75 years with moderately to severely active UC (total Mayo Score [tMS] 6–12, endoscopic subscore ≥2) who had failed ≥1 prior conventional or advanced treatment were randomized to receive RO7790121 SC 50 mg, 150 mg, 450 mg, or matched placebo (PBO) monthly during the 12-week induction period, and RO7790121 SC 50, 150 or 450 mg monthly during the 40-week maintenance period. The primary efficacy endpoint was clinical remission at week 14 by tMS. Secondary endpoints included clinical remission by modified Mayo Score (mMS; aligned with FDA guidance) at weeks 14 and 56, clinical remission by tMS at week 56, endoscopic improvement at weeks 14 and 56, and change from baseline in FCP levels during induction. Safety was assessed throughout the study.
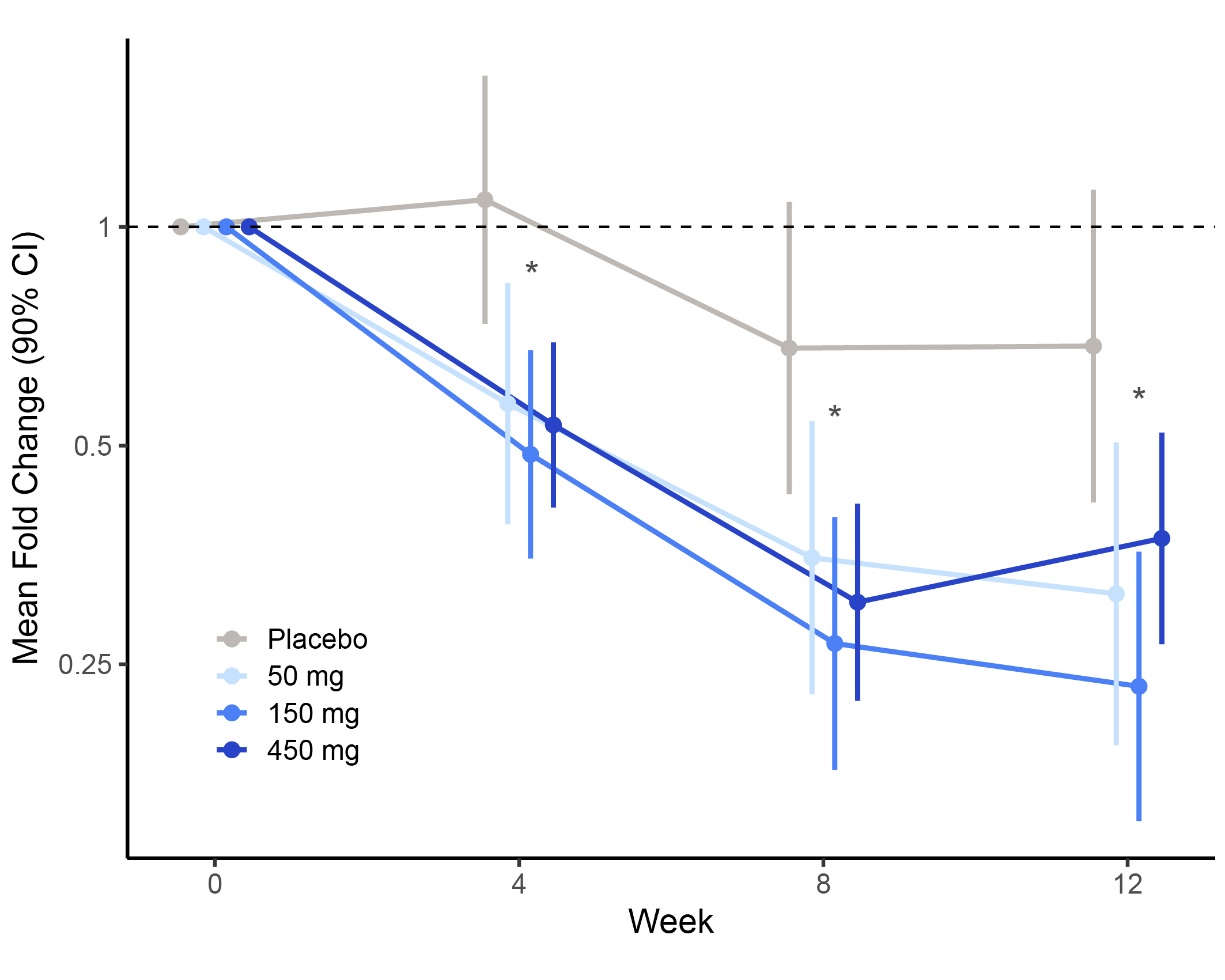
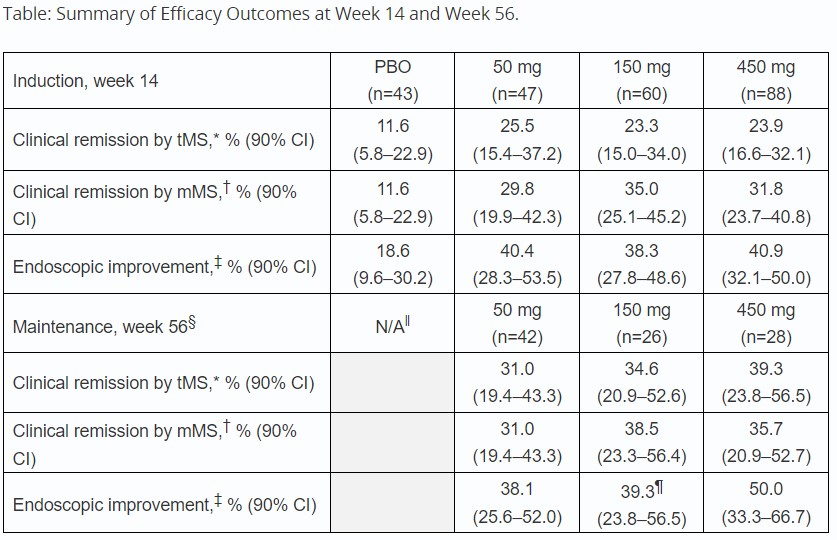
Results: A total of 245 patients received ≥1 dose of RO7790121; 228 patients completed induction and 224 entered maintenance. At week 14, a greater proportion of patients across all treatment doses achieved remission vs PBO by both tMS (p >0.05) and mMS (nominal p< 0.05), sustained through week 56 (Table). Patients receiving any dose of RO7790121 showed greater endoscopic improvement vs PBO at weeks 14 and 56 (Table). Substantial decreases in FCP were observed between baseline and week 12 across all RO7790121 doses vs PBO (Figure). Overall, 47.8% (117/245) of patients during induction experienced ≥1 treatment-emergent adverse event (TEAE), the most common (reported in ≥5% patients overall) were anemia (5.3%) and headache (5.3%). Ten patients experienced serious adverse events in the induction period; 4 PBO, 3 50 mg and 3 450 mg (2 treatment related: 1 PBO, 1 450 mg). There were no treatment discontinuations due to TEAEs.
Discussion: Treatment with RO7790121 resulted in clinical and endoscopic improvements at week 14, which were sustained through week 56, including early decreases in FCP vs PBO. A continuing phase III study will further evaluate these findings.
Clinical trial identification: NCT04090411
Disclosures:
Silvio Danese, MD, PhD1, Jessica R. Allegretti, MD, MPH, FACG2, Stefan Schreiber, MD3, Laurent Peyrin-Biroulet, MD, PhD4, Vipul Jairath, MBChB5, Geert R. D'Haens, MD, PhD6, Jarosław Kierkuś, MD, PhD7, Rupert W. Leong, MD, FRACP8, Andres Yarur, MD9, Jacqueline McBride, PhD10, Daniela Bojic, MD, PhD11, Karen Lasch, MD10, Courtney Schiffman, PhD10, Brian G.. Feagan, MD5, 74, TUSCANY-2: A Dose-Ranging Phase IIb Study Evaluating Efficacy and Safety of RO7790121, an Antibody Against Tumor Necrosis Factor-Like Ligand 1A (Anti-TL1A) in Adults With Moderately to Severely Active Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 2Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 3University Hospital, Kiel, Schleswig-Holstein, Germany; 4INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 5Western University, London, ON, Canada; 6Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands; 7The Children's Memorial Health Institute, Warsaw, Mazowieckie, Poland; 8Concord Hospital and Macquarie University Hospital, Sydney, New South Wales, Australia; 9Cedars-Sinai Medical Center, Los Angeles, CA; 10Genentech, Inc., a member of the Roche Group, South San Francisco, CA; 11F. Hoffmann-La Roche Ltd., Basel, Basel-Stadt, Switzerland
Introduction: RO7790121 (previously PF-06480605, RVT-3101), an anti-TL1A antibody, was effective in a previous open-label phase IIa study for the treatment of active ulcerative colitis (UC). We report results from TUSCANY-2, a phase IIb, randomized, dose-ranging study evaluating efficacy, safety and changes in fecal calprotectin (FCP) levels in patients with moderately to severely active UC receiving subcutaneous (SC) RO7790121.
Methods: In this treat-through study, patients aged 18–75 years with moderately to severely active UC (total Mayo Score [tMS] 6–12, endoscopic subscore ≥2) who had failed ≥1 prior conventional or advanced treatment were randomized to receive RO7790121 SC 50 mg, 150 mg, 450 mg, or matched placebo (PBO) monthly during the 12-week induction period, and RO7790121 SC 50, 150 or 450 mg monthly during the 40-week maintenance period. The primary efficacy endpoint was clinical remission at week 14 by tMS. Secondary endpoints included clinical remission by modified Mayo Score (mMS; aligned with FDA guidance) at weeks 14 and 56, clinical remission by tMS at week 56, endoscopic improvement at weeks 14 and 56, and change from baseline in FCP levels during induction. Safety was assessed throughout the study.
Results: A total of 245 patients received ≥1 dose of RO7790121; 228 patients completed induction and 224 entered maintenance. At week 14, a greater proportion of patients across all treatment doses achieved remission vs PBO by both tMS (p >0.05) and mMS (nominal p< 0.05), sustained through week 56 (Table). Patients receiving any dose of RO7790121 showed greater endoscopic improvement vs PBO at weeks 14 and 56 (Table). Substantial decreases in FCP were observed between baseline and week 12 across all RO7790121 doses vs PBO (Figure). Overall, 47.8% (117/245) of patients during induction experienced ≥1 treatment-emergent adverse event (TEAE), the most common (reported in ≥5% patients overall) were anemia (5.3%) and headache (5.3%). Ten patients experienced serious adverse events in the induction period; 4 PBO, 3 50 mg and 3 450 mg (2 treatment related: 1 PBO, 1 450 mg). There were no treatment discontinuations due to TEAEs.
Discussion: Treatment with RO7790121 resulted in clinical and endoscopic improvements at week 14, which were sustained through week 56, including early decreases in FCP vs PBO. A continuing phase III study will further evaluate these findings.
Clinical trial identification: NCT04090411

Figure: Figure: Mean Fecal Calprotectin Fold Change (μg/g) at Weeks 0, 4, 8 and 12.
*Nominal p value <0.05 in each arm
CI, confidence interval.
*Nominal p value <0.05 in each arm
CI, confidence interval.

Table: Data cut-off: 3 March, 2023.
n=number of participants in the analysis set with observed data or NRI, excluding a total of seven participants with missing data due to medical or operational complications resulting from COVID-19.
*Primary endpoint: defined as tMS ≤2 with no individual subscore >1.
†Secondary endpoint: defined per FDA definition with an mMS 0–2 (endoscopic subscore=0 or 1, ≥1 point decrease from baseline to achieve a stool frequency subscore=0 or 1, and rectal bleeding subscore=0).
‡Defined as endoscopic subscore=0 or 1.
§Analyses were conducted for all dose groups; however, maintenance efficacy results are presented only for patients receiving the same dose during the induction and maintenance periods. Efficacy data from patients who received a lower dose in maintenance vs induction demonstrated sustained efficacy (data not shown).
‖As PBO was not administered during the maintenance period, no data for week 56 are shown.
¶For the endoscopic improvement endpoint at week 56 with the 150 mg dose, data were available for n=28 patients.
CI, confidence interval; COVID-19, coronavirus disease 2019; FDA, Food and Drug Administration; mMS, modified Mayo Score; N/A, non-applicable; NRI, non-responder imputation; PBO, placebo; tMS, total Mayo Score.
n=number of participants in the analysis set with observed data or NRI, excluding a total of seven participants with missing data due to medical or operational complications resulting from COVID-19.
*Primary endpoint: defined as tMS ≤2 with no individual subscore >1.
†Secondary endpoint: defined per FDA definition with an mMS 0–2 (endoscopic subscore=0 or 1, ≥1 point decrease from baseline to achieve a stool frequency subscore=0 or 1, and rectal bleeding subscore=0).
‡Defined as endoscopic subscore=0 or 1.
§Analyses were conducted for all dose groups; however, maintenance efficacy results are presented only for patients receiving the same dose during the induction and maintenance periods. Efficacy data from patients who received a lower dose in maintenance vs induction demonstrated sustained efficacy (data not shown).
‖As PBO was not administered during the maintenance period, no data for week 56 are shown.
¶For the endoscopic improvement endpoint at week 56 with the 150 mg dose, data were available for n=28 patients.
CI, confidence interval; COVID-19, coronavirus disease 2019; FDA, Food and Drug Administration; mMS, modified Mayo Score; N/A, non-applicable; NRI, non-responder imputation; PBO, placebo; tMS, total Mayo Score.
Disclosures:
Silvio Danese: AbbVie – Consultant, Speakers Bureau. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Speakers Bureau. Applied Molecular Transport – Consultant. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion Healthcare – Consultant. Dr Falk Pharma – Consultant. Eli Lilly and Company – Consultant. Enthera – Consultant. Ferring – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. Hospira – Consultant. Inotrem – Consultant. Janssen – Consultant, Speakers Bureau. Johnson & Johnson – Consultant. Morphic – Consultant. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Speakers Bureau. Roche – Consultant. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Speakers Bureau. Teladoc Health – Consultant. TiGenix – Consultant. UCB Inc. – Consultant. Vial – Consultant. Vifor – Consultant.
Jessica R. Allegretti: Abbvie – Consultant, Speakers Bureau. Artugen – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant. Finch Therapeutics – Consultant. Janssen – Consultant. Merck – Grant/Research Support. Pfizer – Consultant. Seres – Consultant.
Stefan Schreiber: AbbVie – Consultant, Personal fees, Speakers Bureau. Amgen – Personal fees. Arena Pharmaceuticals – Consultant, Personal fees, Speakers Bureau. Biogen – Consultant, Personal fees, Speakers Bureau. Bristol Myers Squibb – Consultant, Personal fees, Speakers Bureau. Celgene – Consultant, Personal fees, Speakers Bureau. Celltrion – Consultant, Personal fees, Speakers Bureau. Eli Lilly and Company – Personal fees. Falk – Consultant, Personal fees, Speakers Bureau. Ferring Pharmaceuticals – Personal fees. Fresenius – Consultant, Personal fees, Speakers Bureau. Galapagos – Personal fees. Gilead – Consultant, Personal fees. Hikma Pharmaceuticals – Advisory Committee/Board Member, Consultant. I-MAB – Consultant, Personal fees. Janssen – Consultant, Personal fees, Speakers Bureau. Morphic – Personal fees. MSD – Consultant, Personal fees, Speakers Bureau. Mylan – Consultant, Personal fees. Novartis – Personal fees. Pfizer Inc – Consultant, Personal fees, Speakers Bureau. Protagonist – Consultant, Personal fees. Provention Bio – Consultant, Personal fees. Roche – Personal fees. Sandoz/Hexal – Personal fees. Shire – Personal fees. Takeda – Consultant, Personal fees, Speakers Bureau. Theravance Biopharma – Consultant, Personal fees. Ventyx – Consultant, Personal fees.
Laurent Peyrin-Biroulet: AbbVie – Grant/Research Support, Personal fees. Allergan – Personal Fees. Alma Bio Therapeutics – Personal Fees. Amgen – Personal Fees. Applied Molecular Transport – Personal Fees. Arena – Personal Fees. Biogen – Personal Fees. Boehringer Ingelheim – Personal Fees. Bristol Myers Squibb – Personal Fees. Celgene – Personal Fees. Celltrion – Personal Fees. CTMA – Stock Options. Enterome – Personal Fees. Enthera – Personal Fees. Ferring – Personal Fees. Fresenius Kabi – Personal Fees. Genentech – Personal Fees. Gilead – Personal Fees. Hikma – Personal Fees. InDex Pharmaceuticals – Personal Fees. Janssen – Personal Fees. Lilly – Personal Fees. MSD – Grant/Research Support, Personal Fees. Mylan – Personal Fees. Nestlé – Personal Fees. Norgine – Personal Fees. Oppilan Pharma – Personal Fees. OSE Immunotherapeutics – Personal Fees. Pfizer Inc – Personal Fees. Pharmacosmos – Fees. Samsung Bioepis – Personal Fees. Sandoz – Personal Fees. Sterna – Personal Fees. Sublimity Therapeutics – Personal Fees. Takeda – Grant/Research Support, Personal Fees. Tillotts – Personal Fees. Vifor – Personal Fees.
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Employee, Grant/Research Support, Speakers Bureau. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Advisory Committee/Board Member, Consultant. Enthera – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Advisory Committee/Board Member, Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Advisory Committee/Board Member, Consultant. JAMP – Advisory Committee/Board Member, Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. London Health Sciences Centre – Employee. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Advisory Committee/Board Member, Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. SCOPE – Advisory Committee/Board Member, Consultant. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Synedgen – Advisory Committee/Board Member, Consultant. Takeda – Consultant, Grant/Research Support, Speakers Bureau. TD Securities – Advisory Committee/Board Member, Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Geert D'Haens: AbbVie – Advisor or Review Panel Member, Speakers Bureau. Agomab Therapeutics – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. Allergan – Advisor or Review Panel Member. Alphabiomics – Advisor or Review Panel Member. AstraZeneca – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Speakers Bureau. Ferring – Advisor or Review Panel Member. Galapagos – Advisor or Review Panel Member, Speakers Bureau. GlaxoSmithKline – Advisor or Review Panel Member. Immunic – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Seres – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau. Tillotts – Advisor or Review Panel Member, Speakers Bureau. Ventyx – Advisor or Review Panel Member.
Jarosław Kierkuś: Bristol-Myers Squibb – Fees. Celltrion – Fees. Ferring – Fees. Fresenius – Fees. Janssen – Fees. Lilly – Fees. Nestlé – Fees.
Rupert W. Leong: Abbvie – Advisory Committee/Board Member. Aspen – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Celgene – Advisory Committee/Board Member. Cellitron – Advisory Committee/Board Member, Grant/Research Support. Chiesi – Advisory Committee/Board Member. Ferring – Advisory Committee/Board Member. Gastroenterological Society of Australia – Grant/Research Support. Glutagen – Advisory Committee/Board Member. Gutsy Group – Grant/Research Support. Hospira – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support. Joanna Tiddy grant – Grant/Research Support. Lilly – Advisory Committee/Board Member. McKusker Charitable Foundation – Grant/Research Support. MSD – Advisory Committee/Board Member. NHMRC – Grant/Research Support. Novartis – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Grant/Research Support. Prometheus Biosciences – Advisory Committee/Board Member. Shire – Grant/Research Support. Takeda – Advisory Committee/Board Member, Grant/Research Support.
Andres Yarur: AbbVie – Consultant, served on clinical trial steering committee. Abivax – Advisory Committee/Board Member, Consultant. Arena – Consultant, served on clinical trial steering committee. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Consultant, served on clinical trial steering committee. Celltrion – Consultant, served on clinical trial steering committee. Johnson and Johnson – Advisory Committee/Board Member, Consultant. Pfizer – Consultant, served on clinical trial steering committee. Takeda – Consultant, served on clinical trial steering committee.
Jacqueline McBride: Roche/Genentech – Employee, Stock or Other Ownership Interests.
Daniela Bojic: F. Hoffmann-La Roche – Employee.
Karen Lasch: Genentech/Roche – Employee.
Courtney Schiffman: Genentech/Roche – Employee, Stock or Other Ownership Interests.
Brian Feagan: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. AbolerIS – Consultant. AgomAB Therapeutics – Consultant. Allianthera – Consultant. Amgen – Advisory Committee/Board Member, Consultant. AnaptysBio – Advisory Committee/Board Member, Consultant. Applied Molecular Transport Inc – Advisory Committee/Board Member, Consultant. Arena Pharma – Consultant. Atomwise – Consultant. Avoro Capital Advisors – Consultant. Axio Research – Advisory Committee/Board Member. BioJamp – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Boxer – Consultant. Celgene/Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Celsius Therapeutics – Consultant. Connect BioPharma – Consultant, stock or other ownership interest. Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1 Capital – Advisory Committee/Board Member, Consultant. Equillium – Consultant. Ermium – Consultant. First Wave – Consultant. First Word Group – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Advisory Committee/Board Member, Consultant. Gilead – Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Gossamer Pharma – Consultant, Stock Options. Hinge Bio – Consultant. Hot Spot Therapeutics – Consultant. Imhotex – Consultant. Immunic Therapeutics – Consultant. InDex Pharmaceuticals – Advisory Committee/Board Member, Consultant. JAKAcademy – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. L.E.K. Consulting – Consultant. Landos Biopharma – Consultant. Leadiant – Consultant. Lenczner Slaght – Consultant, payment for expert testimony. LifeSci Capital – Consultant. Lilly – Advisory Committee/Board Member, Consultant. Lument AB – Consultant. Millennium – Consultant. MiroBio – Advisory Committee/Board Member, Consultant. Morgan Lewis – Consultant, payment for expert testimony. Morphic Therapeutics – Advisory Committee/Board Member, Consultant. Mylan – Consultant. OM Pharma – Consultant. Origo BioPharma – Advisory Committee/Board Member, Consultant. Orphagen – Consultant. Pandion Therapeutics – Consultant. Pendopharm – Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Grant/Research Support. Play to Know AG – Consultant. Progenity – Advisory Committee/Board Member, Consultant. Prometheus – Advisory Committee/Board Member, Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. REDX – Advisory Committee/Board Member, Consultant. Roche – Consultant. Sandoz – Consultant. Sanofi – Advisory Committee/Board Member, Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Surrozen Inc. – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Teva – Advisory Committee/Board Member, Consultant. Thelium – Consultant. Tigenix – Consultant. Tillotts Pharma – Advisory Committee/Board Member, Consultant. Ventyx Biosciences – Consultant. VHSquared Ltd – Consultant. Viatris – Consultant. Ysios – Consultant. Ysopia – Consultant. Zealand Pharma – Consultant.
Silvio Danese, MD, PhD1, Jessica R. Allegretti, MD, MPH, FACG2, Stefan Schreiber, MD3, Laurent Peyrin-Biroulet, MD, PhD4, Vipul Jairath, MBChB5, Geert R. D'Haens, MD, PhD6, Jarosław Kierkuś, MD, PhD7, Rupert W. Leong, MD, FRACP8, Andres Yarur, MD9, Jacqueline McBride, PhD10, Daniela Bojic, MD, PhD11, Karen Lasch, MD10, Courtney Schiffman, PhD10, Brian G.. Feagan, MD5, 74, TUSCANY-2: A Dose-Ranging Phase IIb Study Evaluating Efficacy and Safety of RO7790121, an Antibody Against Tumor Necrosis Factor-Like Ligand 1A (Anti-TL1A) in Adults With Moderately to Severely Active Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


