Oral Paper Presentation
Annual Scientific Meeting
Session: Presidential Plenary Session 1
4 - Heartburn Frequency and Symptom Improvement Rates of Treated Episodes when Switching From Daily to On-Demand Vonoprazan Treatment for Non-Erosive Reflux Disease
Monday, October 28, 2024
8:36 AM - 8:48 AM ET
Location: Terrace Ballroom

Ronnie Fass, MD, MACG (he/him/his)
MetroHealth Medical Center
Orange, OH
Presenting Author(s)
Award: ACG Outstanding Research Award in the Esophagus Category
Ronnie Fass, MD1, Joan W. Chen, MD, MS2, Joel J. Heidelbaugh, MD, FACG3, Anish Patel, DO4, Reena V. Chokshi, MD5, Thomas Harris, BSc, PharmD6, Hillary Graham, MS6, Eckhard Leifke, MD6, David Armstrong, MA, MBBCh7
1MetroHealth Medical Center, Case Western Reserve University, Cleveland, OH; 2University of Michigan, Ann Arbor, MI; 3University of Michigan Medical School, Ann Arbor, MI; 4Brooke Army Medical Center, Fort Sam Houston, TX; 5Baylor College of Medicine, Houston, TX; 6Phathom Pharmaceuticals, Buffalo Grove, IL; 7McMaster University, Hamilton, ON, Canada
Introduction: The efficacy and safety of on-demand vonoprazan, a potassium-competitive acid blocker, was evaluated against placebo in patients with endoscopy-proven non-erosive reflux disease (NERD). The aim of this study is to evaluate the frequency of heartburn (HB) when switching to on-demand treatment after symptom control and the rate of symptom improvement of treated episodes.
Methods: This was a post hoc analysis of a double-blind, randomized, placebo-controlled trial (NCT04799158). Patients completing a run-in period of daily vonoprazan 20 mg for 4-weeks were eligible for the 6-week on-demand period if they were 80% compliant with open-label study drug and electronic diary entries and reported no HB during the last seven days of the run-in period. Patients were randomized to one of four treatment arms (vonoprazan 10 mg, 20 mg, or 40 mg, placebo) and prompted to take study drug when experiencing HB. HB severity (none, mild, moderate, severe, very severe) was reported at time of treatment and 0.5, 1, 1.5, 2 and 3 hours after taking study drug. Improvement in severity was defined as a minimum of one grade reduction from the initial severity.
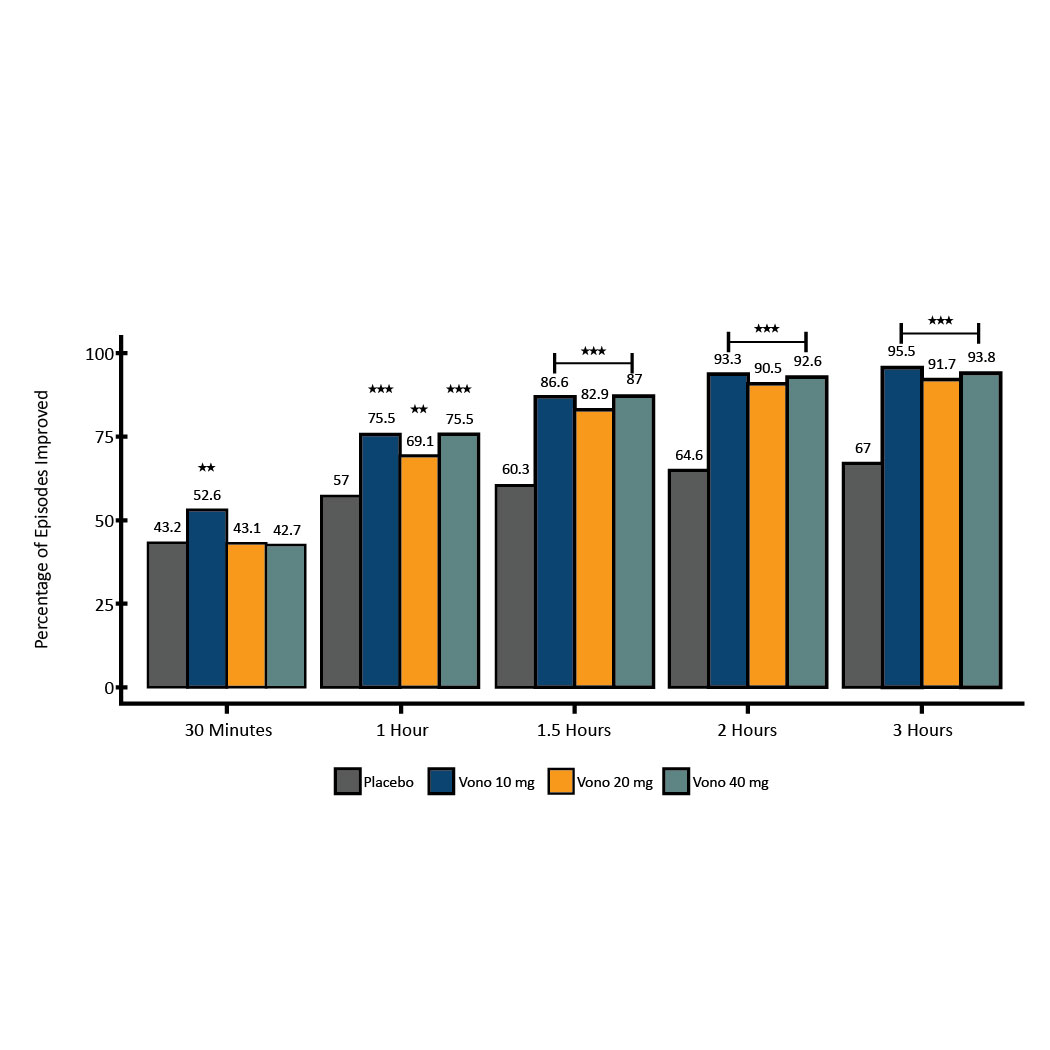
Results: Patients eligible for the on-demand period (n=207) reported a mean of 16.1% [95% CI: (13.5%, 18.7%)] HB-free days during screening. HB-free days increased during the run-in period to a mean of 82.9% [95% CI: (80.4%, 85.4%)]. When transitioning to on-demand treatment, the percentage of HB-free days remained well above pre-treatment levels for all treatments, ranging from 71% to 75%. These results were consistent through the 6-week treatment period. The difference in percentage of treated HB episodes that improved between the active and placebo groups was evident within the first hour (10 mg [75.5%, p<0.0001], 20 mg [69.1%, p = 0.0010], 40 mg [75.5%, p<0.0001], placebo [57.0%], Figure 1). Over 90% of episodes treated with vonoprazan improved at two hours.
Discussion: Following effective symptom management with daily vonoprazan treatment, a high rate of HB-free days was sustained over 6-weeks of on-demand treatment. Upon treatment of episodes, improvement of HB occurred rapidly with differences observed within one hour in vonoprazan-treated episodes compared to placebo. These results suggest that the initial daily run-in treatment was effective and that on-demand vonoprazan may be a suitable option in patients who respond to continuous treatment, with improvement in severity occurring within one hour.
Disclosures:
Ronnie Fass, MD1, Joan W. Chen, MD, MS2, Joel J. Heidelbaugh, MD, FACG3, Anish Patel, DO4, Reena V. Chokshi, MD5, Thomas Harris, BSc, PharmD6, Hillary Graham, MS6, Eckhard Leifke, MD6, David Armstrong, MA, MBBCh7, 4, Heartburn Frequency and Symptom Improvement Rates of Treated Episodes when Switching From Daily to On-Demand Vonoprazan Treatment for Non-Erosive Reflux Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Ronnie Fass, MD1, Joan W. Chen, MD, MS2, Joel J. Heidelbaugh, MD, FACG3, Anish Patel, DO4, Reena V. Chokshi, MD5, Thomas Harris, BSc, PharmD6, Hillary Graham, MS6, Eckhard Leifke, MD6, David Armstrong, MA, MBBCh7
1MetroHealth Medical Center, Case Western Reserve University, Cleveland, OH; 2University of Michigan, Ann Arbor, MI; 3University of Michigan Medical School, Ann Arbor, MI; 4Brooke Army Medical Center, Fort Sam Houston, TX; 5Baylor College of Medicine, Houston, TX; 6Phathom Pharmaceuticals, Buffalo Grove, IL; 7McMaster University, Hamilton, ON, Canada
Introduction: The efficacy and safety of on-demand vonoprazan, a potassium-competitive acid blocker, was evaluated against placebo in patients with endoscopy-proven non-erosive reflux disease (NERD). The aim of this study is to evaluate the frequency of heartburn (HB) when switching to on-demand treatment after symptom control and the rate of symptom improvement of treated episodes.
Methods: This was a post hoc analysis of a double-blind, randomized, placebo-controlled trial (NCT04799158). Patients completing a run-in period of daily vonoprazan 20 mg for 4-weeks were eligible for the 6-week on-demand period if they were 80% compliant with open-label study drug and electronic diary entries and reported no HB during the last seven days of the run-in period. Patients were randomized to one of four treatment arms (vonoprazan 10 mg, 20 mg, or 40 mg, placebo) and prompted to take study drug when experiencing HB. HB severity (none, mild, moderate, severe, very severe) was reported at time of treatment and 0.5, 1, 1.5, 2 and 3 hours after taking study drug. Improvement in severity was defined as a minimum of one grade reduction from the initial severity.
Results: Patients eligible for the on-demand period (n=207) reported a mean of 16.1% [95% CI: (13.5%, 18.7%)] HB-free days during screening. HB-free days increased during the run-in period to a mean of 82.9% [95% CI: (80.4%, 85.4%)]. When transitioning to on-demand treatment, the percentage of HB-free days remained well above pre-treatment levels for all treatments, ranging from 71% to 75%. These results were consistent through the 6-week treatment period. The difference in percentage of treated HB episodes that improved between the active and placebo groups was evident within the first hour (10 mg [75.5%, p<0.0001], 20 mg [69.1%, p = 0.0010], 40 mg [75.5%, p<0.0001], placebo [57.0%], Figure 1). Over 90% of episodes treated with vonoprazan improved at two hours.
Discussion: Following effective symptom management with daily vonoprazan treatment, a high rate of HB-free days was sustained over 6-weeks of on-demand treatment. Upon treatment of episodes, improvement of HB occurred rapidly with differences observed within one hour in vonoprazan-treated episodes compared to placebo. These results suggest that the initial daily run-in treatment was effective and that on-demand vonoprazan may be a suitable option in patients who respond to continuous treatment, with improvement in severity occurring within one hour.

Figure: Figure 1. Percentage of heartburn episodes improved at each time point over three hours after treatment. * p < 0.1, ** p < 0.05, *** p < 0.0001, compared with placebo.
Disclosures:
Ronnie Fass: Braintree laboratories/Sebela – Advisor or Review Panel Member. carnot – Advisor or Review Panel Member, Speaker. Daewoong – Speaker. Dexcal – Advisor or Review Panel Member. Eisai, Inc. – speaker. GERDCare/Celexio – Advisor or Review Panel Member. Intra-Sana – Speaker. Medicamenta – Speaker. Medtronic – Advisor or Review Panel Member. Phathom Pharmaceuticals – Advisor or Review Panel Member. Restech – Stock Options. Syneos – Advisor or Review Panel Member. Takeda – Speaker.
Joan Chen: Phathom Pharmaceuticals – Consultant.
Joel Heidelbaugh indicated no relevant financial relationships.
Anish Patel: Abbvie – Consultant. BMS – Speakers Bureau. Eli Lily – Speakers Bureau. Janssen – Speakers Bureau. Pfizer – Speakers Bureau. Phathom Pharmaceuticals – Speakers Bureau. Takeda – Speakers Bureau.
Reena Chokshi: Enterra – Consultant. Medtronic – Consultant.
Thomas Harris: Phathom Pharmaceuticals – Employee, Owner/Ownership Interest, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Hillary Graham: Phathom Pharmaceuticals – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Eckhard Leifke: Phathom Pharmaceuticals – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
David Armstrong: AI VALI Inc – Owner/Ownership Interest. Amgen – Speakers Bureau. Cinclus Pharma – Advisor or Review Panel Member. Fresenius Kabi – Speakers Bureau. Phathom Pharmaceuticals – Advisor or Review Panel Member. Sanofi – Advisor or Review Panel Member. Takeda – Speakers Bureau.
Ronnie Fass, MD1, Joan W. Chen, MD, MS2, Joel J. Heidelbaugh, MD, FACG3, Anish Patel, DO4, Reena V. Chokshi, MD5, Thomas Harris, BSc, PharmD6, Hillary Graham, MS6, Eckhard Leifke, MD6, David Armstrong, MA, MBBCh7, 4, Heartburn Frequency and Symptom Improvement Rates of Treated Episodes when Switching From Daily to On-Demand Vonoprazan Treatment for Non-Erosive Reflux Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.



