Back
Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 2A - Small Intestine / Functional / Liver
19 - Phase 3, Double-Blind, Randomized STARS Trial of Apraglutide Once-Weekly in Patients with Short Bowel Syndrome and Intestinal Failure (SBS-IF): Subgroup Analyses by Baseline Demographics and SBS Disease Characteristics
Tuesday, October 29, 2024
8:30 AM – 8:40 AM ET
Location: Terrace Ballroom 1

Kishore Iyer, MD, MBBS
Mount Sinai Medical Center
New York, NY
Presenting Author(s)
Kishore Iyer, MD, MBBS1, Francisca Joly, MD, PhD2, Donald Kirby, MD3, Simon Lal, MD, PhD4, Kelly Tappenden, PhD, RD5, Palle Jeppesen, MD, PhD6, Nader N Youssef, MD, MBA, FACG7, Mena Boules, MD8, Chang Ming, MS, PhD7, Tomasz Masior, MD7, Susanna Huh, MD, MPH7, Tim Vanuytsel, MD, PhD9
1Mount Sinai Medical Center, New York, NY; 2Centre for Intestinal Failure, Hôpital Beaujon, Paris, Ile-de-France, France; 3Cleveland Clinic, Cleveland, OH; 4University of Manchester Manchester, Manchester, England, United Kingdom; 5The University of Utah, Salt Lake City, UT; 6Rigshospitalet, Copenhagen University Hospital, Copenhagen, Sjelland, Denmark; 7Ironwood Pharmaceuticals, Boston, MA; 8Ironwood Pharmaceuticals, Inc., Boston, MA; 9Leuven Intestinal Failure and Transplantation Center, University Hospital, Leuven, Brussels Hoofdstedelijk Gewest, Belgium
Introduction: Patients (pts) with SBS-IF depend on parenteral support (PS) to meet nutritional and/or fluid requirements, based on remnant bowel anatomy. Apraglutide (APRA), a long-acting glucagon-like peptide-2 (GLP-2) analog administered subcutaneously (SC) once-weekly, stimulates intestinal adaptation and reduce PS requirements, as shown in the pivotal STARS trial. Here we present subgroup analyses from this trial evaluating the treatment effect of APRA vs placebo (PBO) by baseline (BL) demographic and SBS disease-specific characteristics.
Methods: STARS was a global, Phase 3, double-blind, PBO-controlled trial (NCT04627025) evaluating efficacy and safety of APRA (5 mg if pt ≥50 kg, 2.5 mg if pt < 50kg) or matching PBO (randomization 2:1), stratified by residual intestinal anatomy (stoma or colon-in-continuity). The primary endpoint was relative change from BL in actual PS weekly volume at Week (Wk) 24 in the overall population. Reduction of additional ≥1 day/wk off PS from BL was a key secondary endpoint. This subgroup analysis examined the primary endpoint by demographics: gender, age (< 65 and ≥65 years old), body weight (< 50 and ≥50 years old), region (EU, USA, or rest of the world), race (Asian, Caucasian, or other), ethnicity (Hispanic/Latino or not); and SBS disease characteristics: PS volume (< 12 and ≥12 L), length of remnant small intestine (< 80 cm and ≥80 cm), and time from SBS diagnosis (< 65.7 and ≥65.7 months) at BL. Analyses used a mixed effect model for repeated measures (MMRM).
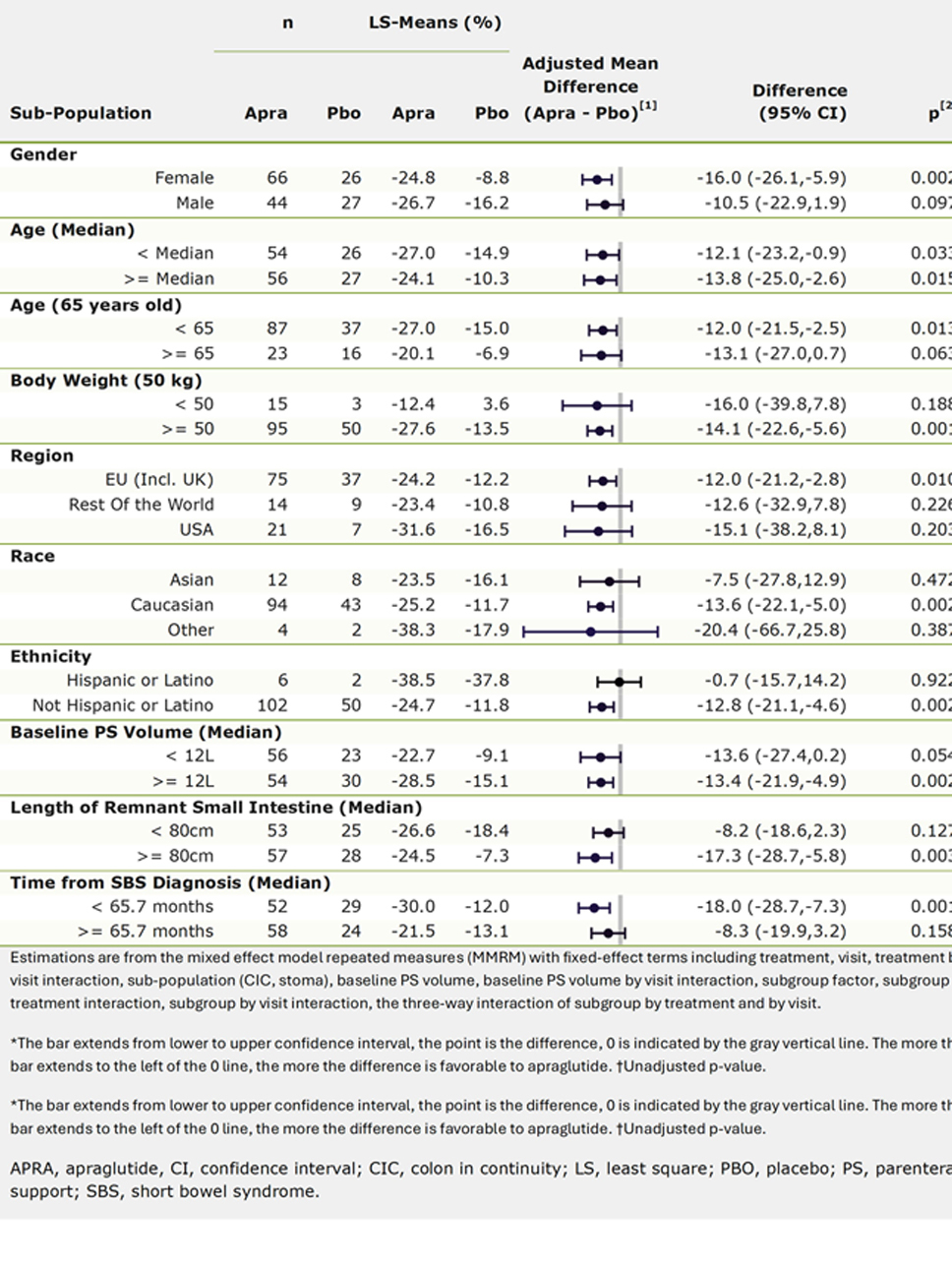
Results: The primary endpoint was met at Wk 24 (n=163; overall population), with a significantly larger reduction in weekly PS volume from BL with APRA vs PBO (-25.5% vs -12.5%, p=0.001). These results have been presented previously. Subgroup analyses showed consistent treatment effect for APRA vs PBO across all subgroups in the overall population (Figure 1). A similar magnitude of effect was observed regardless of body weight, with differences of -16.0% (p=0.188) and -14.1% (p=0.001) in pts weighing < 50 kg (reflects the 2.5 mg dose) and ≥50 kg (reflects the 5 mg dose). Similar magnitude of effect was also seen regardless of whether PS volume at BL was < 12L or ≥12L (-13.6% [p=0.054] and -13.4% [p=0.002]).
Discussion: In the overall population of the STARS trial, subgroup analyses of the primary endpoint by BL demographics and SBS disease characteristics showed consistent treatment effect of APRA.
Disclosures:
Kishore Iyer, MD, MBBS1, Francisca Joly, MD, PhD2, Donald Kirby, MD3, Simon Lal, MD, PhD4, Kelly Tappenden, PhD, RD5, Palle Jeppesen, MD, PhD6, Nader N Youssef, MD, MBA, FACG7, Mena Boules, MD8, Chang Ming, MS, PhD7, Tomasz Masior, MD7, Susanna Huh, MD, MPH7, Tim Vanuytsel, MD, PhD9, 19, Phase 3, Double-Blind, Randomized STARS Trial of Apraglutide Once-Weekly in Patients with Short Bowel Syndrome and Intestinal Failure (SBS-IF): Subgroup Analyses by Baseline Demographics and SBS Disease Characteristics, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Mount Sinai Medical Center, New York, NY; 2Centre for Intestinal Failure, Hôpital Beaujon, Paris, Ile-de-France, France; 3Cleveland Clinic, Cleveland, OH; 4University of Manchester Manchester, Manchester, England, United Kingdom; 5The University of Utah, Salt Lake City, UT; 6Rigshospitalet, Copenhagen University Hospital, Copenhagen, Sjelland, Denmark; 7Ironwood Pharmaceuticals, Boston, MA; 8Ironwood Pharmaceuticals, Inc., Boston, MA; 9Leuven Intestinal Failure and Transplantation Center, University Hospital, Leuven, Brussels Hoofdstedelijk Gewest, Belgium
Introduction: Patients (pts) with SBS-IF depend on parenteral support (PS) to meet nutritional and/or fluid requirements, based on remnant bowel anatomy. Apraglutide (APRA), a long-acting glucagon-like peptide-2 (GLP-2) analog administered subcutaneously (SC) once-weekly, stimulates intestinal adaptation and reduce PS requirements, as shown in the pivotal STARS trial. Here we present subgroup analyses from this trial evaluating the treatment effect of APRA vs placebo (PBO) by baseline (BL) demographic and SBS disease-specific characteristics.
Methods: STARS was a global, Phase 3, double-blind, PBO-controlled trial (NCT04627025) evaluating efficacy and safety of APRA (5 mg if pt ≥50 kg, 2.5 mg if pt < 50kg) or matching PBO (randomization 2:1), stratified by residual intestinal anatomy (stoma or colon-in-continuity). The primary endpoint was relative change from BL in actual PS weekly volume at Week (Wk) 24 in the overall population. Reduction of additional ≥1 day/wk off PS from BL was a key secondary endpoint. This subgroup analysis examined the primary endpoint by demographics: gender, age (< 65 and ≥65 years old), body weight (< 50 and ≥50 years old), region (EU, USA, or rest of the world), race (Asian, Caucasian, or other), ethnicity (Hispanic/Latino or not); and SBS disease characteristics: PS volume (< 12 and ≥12 L), length of remnant small intestine (< 80 cm and ≥80 cm), and time from SBS diagnosis (< 65.7 and ≥65.7 months) at BL. Analyses used a mixed effect model for repeated measures (MMRM).
Results: The primary endpoint was met at Wk 24 (n=163; overall population), with a significantly larger reduction in weekly PS volume from BL with APRA vs PBO (-25.5% vs -12.5%, p=0.001). These results have been presented previously. Subgroup analyses showed consistent treatment effect for APRA vs PBO across all subgroups in the overall population (Figure 1). A similar magnitude of effect was observed regardless of body weight, with differences of -16.0% (p=0.188) and -14.1% (p=0.001) in pts weighing < 50 kg (reflects the 2.5 mg dose) and ≥50 kg (reflects the 5 mg dose). Similar magnitude of effect was also seen regardless of whether PS volume at BL was < 12L or ≥12L (-13.6% [p=0.054] and -13.4% [p=0.002]).
Discussion: In the overall population of the STARS trial, subgroup analyses of the primary endpoint by BL demographics and SBS disease characteristics showed consistent treatment effect of APRA.

Figure: Table 1. Subgroup analyses of the primary endpoint relative change in parenteral support at Week 24 by baseline demographics and SBS disease characteristics (full analysis set)
Disclosures:
Kishore Iyer: Hanmi Pharmaceuticals – Advisor or Review Panel Member. Northsea Therapeutics – Advisor or Review Panel Member, Grant/Research Support. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Advisor or Review Panel Member, Grant/Research Support.
Francisca Joly: Baxter – Grant/Research Support, Lecturer. BBraun – Lecturer. Carembouche – Advisor or Review Panel Member, Consultant, Grant/Research Support. Fresenius Kabi – Lecturer. Hanmi Pharmaceuticals – Advisor or Review Panel Member, Consultant. Ironwood Pharmaceuticals – Advisor or Review Panel Member, Consultant. Janssen and Janssen – Lecturer. Mobile3esolutions – Advisor or Review Panel Member, Consultant, Grant/Research Support. NorthSea Therapeutics – Advisor or Review Panel Member, Consultant. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Grant/Research Support. Theradial – Lecturer. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Advisor or Review Panel Member, Consultant, Grant/Research Support. Zealand Pharma – Advisor or Review Panel Member, Consultant, Grant/Research Support.
Donald Kirby: OWYN – Advisor or Review Panel Member, Consultant. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Advisor or Review Panel Member, Consultant.
Simon Lal: B Braun – Honoraria and/or educational support. Baxter – Grant/Research Support, Honoraria and/or educational support. Fresenius Kabi – Grant/Research Support, Honoraria and/or educational support. Northsea – Honoraria and/or educational support. Takeda Pharmaceuticals – Grant/Research Support, Honoraria and/or educational support. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Honoraria and/or educational support. Zealand Pharma – Honoraria and/or educational support.
Kelly Tappenden: Abbott Nutrition Health Institute – Advisor or Review Panel Member, Advisory Committee/Board Member. Nutricia North America – Advisor or Review Panel Member, Advisory Committee/Board Member. OWYN – Consultant. Takeda Pharmaceuticals – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Palle Jeppesen: Albumedix A/S – Honoraria, educational and/or grants. ArTara Therapeutics – Honoraria, educational and/or grants. Bainan Biotech – Honoraria, educational and/or grants. Baxter – Honoraria, educational and/or grants. Coloplast A/S – Honoraria, educational and/or grants. Ferring Pharmaceuticals – Honoraria, educational and/or grants. Fresenius Kabi – Honoraria, educational and/or grants. GlyPharma Therapeutic – Honoraria, educational and/or grants. Hanmi Pharmaceuticals – Honoraria, educational and/or grants. Ironwood Pharmaceuticals – Honoraria, educational and/or grants. Naia Pharmaceuticals – Honoraria, educational and/or grants. NPS Pharmaceuticals – Honoraria, educational and/or grants. Protara Therapeutics – Honoraria, educational and/or grants. Shire pharmaceuticals – Honoraria, educational and/or grants. Takeda pharmaceuticals – Honoraria, educational and/or grants. The Novo Nordisk Foundation – Honoraria, educational and/or grants. Therachon – Honoraria, educational and/or grants. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Honoraria, educational and/or grants. Zealand Pharma – Honoraria, educational and/or grants.
Nader N Youssef: Ironwood Pharmaceuticals – Employee.
Mena Boules: Ironwood Pharmaceuticals – Employee, Stock-publicly held company(excluding mutual/index funds).
Chang Ming: Ironwood Pharmaceuticals – Employee.
Tomasz Masior: Ironwood Pharmaceuticals – Employee.
Susanna Huh: Ironwood Pharmaceuticals – Employee.
Tim Vanuytsel: Abbott Nutrition Health Institute – Lecturer. Baxter – Consultant, Lecturer. Biocodex – Lecturer. BMS – Consultant. Danone – Consultant. Dr. Falk Pharma – Consultant, Lecturer. Fresenius Kabi – Lecturer. Ipsen, Menarini – Lecturer. MyHealth – Grant/Research Support, Lecturer. North Sea Therapeutics – Consultant. Remedus – Lecturer. Takeda Pharmaceuticals – Consultant, Grant/Research Support, Lecturer. Truvion – Consultant, Lecturer. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Consultant, Grant/Research Support, Lecturer.
Kishore Iyer, MD, MBBS1, Francisca Joly, MD, PhD2, Donald Kirby, MD3, Simon Lal, MD, PhD4, Kelly Tappenden, PhD, RD5, Palle Jeppesen, MD, PhD6, Nader N Youssef, MD, MBA, FACG7, Mena Boules, MD8, Chang Ming, MS, PhD7, Tomasz Masior, MD7, Susanna Huh, MD, MPH7, Tim Vanuytsel, MD, PhD9, 19, Phase 3, Double-Blind, Randomized STARS Trial of Apraglutide Once-Weekly in Patients with Short Bowel Syndrome and Intestinal Failure (SBS-IF): Subgroup Analyses by Baseline Demographics and SBS Disease Characteristics, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

