Tuesday Poster Session
Category: Liver
P4805 - Pembrolizumab-Induced Acute Liver Failure in a Patient With Stage IV Recurrent Uterine Cancer
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Mena Saad, DO
Riverside University Health System
Moreno Valley, CA
Presenting Author(s)
Mena Saad, DO1, Danyal O. Imam, MBBS1, Sharanya Kumar, MD1, Harold J. Duarte, MD2, Jun Song, MD3, Wichit Srikureja, MD1, Ronaldo Gnass, MD1, Jeremy Deisch, MD1, Steve Serrao, MD1
1Riverside University Health System, Moreno Valley, CA; 2Loma Linda University Health, Loma Linda, CA; 3Loma Linda University Medical Center, Loma Linda, CA
Introduction: Pembrolizumab is an FDA-approved immune checkpoint inhibitor (ICI) that is approved for the treatment of various cancers including uterine cancer. The most common side effects include fatigue, rash, and diarrhea but can rarely cause more serious effects including severe hepatotoxicity and liver failure. We present a case of pembrolizumab-induced acute liver failure.
Case Description/Methods: The patient is a 66-year-old female with a history of recurrent metastatic endometrial endometrioid adenocarcinoma status post total hysterectomy who was receiving immunotherapy with pembrolizumab. She presented with nausea, emesis, and right upper quadrant abdominal pain. Vital signs were significant for a temperature of 101F but were otherwise within normal limits. Laboratory studies showed AST 3230, ALT 2449, Alkaline phosphatase 443, Total bilirubin 4.0, INR 1.0. Viral hepatitis workup was unrevealing and abdominal ultrasound showed gallbladder wall thickening, pericholecystic fluid, and CBD dilation of 7.7mm with sludge visualized within the CBD but no stones visualized. Endoscopic retrograde cholangiopancreatography was unremarkable. Liver biopsies were taken during subsequent endoscopic ultrasound. Pathology was consistent with ICI hepatitis. Pembrolizumab therapy was discontinued and she was started on pulse dose steroids and transitioned to oral steroids with marked improvement in her liver enzymes upon discharge. One week later, she returned to the emergency department with similar presenting symptoms and encephalopathy. Laboratory studies were significant for AST >5000, ALT 9203, Alkaline phosphatase 443, Total bilirubin 7.4, INR 2.2. She was started on steroids and N-acetylcysteine for non-acetaminophen-induced acute liver failure. Despite these therapies, the patient clinically deteriorated with worsening liver enzymes from acute liver failure.
Discussion: This case highlights the importance of recognizing ICI as a potential cause of severe hepatotoxicity and acute liver failure. Pembrolizumab, although historically associated with a lower incidence of ICI hepatitis, can lead to severe hepatotoxicity. In addition to stopping pembrolizumab, treatment with IV steroids and avoidance of other medications with potential liver toxicity is important to avoid worsening liver failure but liver transplant evaluation is of utmost importance to improve survival. Delayed presentation of fulminant liver failure can be long-term sequelae of immune checkpoint inhibitor use.

Disclosures:
Mena Saad, DO1, Danyal O. Imam, MBBS1, Sharanya Kumar, MD1, Harold J. Duarte, MD2, Jun Song, MD3, Wichit Srikureja, MD1, Ronaldo Gnass, MD1, Jeremy Deisch, MD1, Steve Serrao, MD1. P4805 - Pembrolizumab-Induced Acute Liver Failure in a Patient With Stage IV Recurrent Uterine Cancer, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Riverside University Health System, Moreno Valley, CA; 2Loma Linda University Health, Loma Linda, CA; 3Loma Linda University Medical Center, Loma Linda, CA
Introduction: Pembrolizumab is an FDA-approved immune checkpoint inhibitor (ICI) that is approved for the treatment of various cancers including uterine cancer. The most common side effects include fatigue, rash, and diarrhea but can rarely cause more serious effects including severe hepatotoxicity and liver failure. We present a case of pembrolizumab-induced acute liver failure.
Case Description/Methods: The patient is a 66-year-old female with a history of recurrent metastatic endometrial endometrioid adenocarcinoma status post total hysterectomy who was receiving immunotherapy with pembrolizumab. She presented with nausea, emesis, and right upper quadrant abdominal pain. Vital signs were significant for a temperature of 101F but were otherwise within normal limits. Laboratory studies showed AST 3230, ALT 2449, Alkaline phosphatase 443, Total bilirubin 4.0, INR 1.0. Viral hepatitis workup was unrevealing and abdominal ultrasound showed gallbladder wall thickening, pericholecystic fluid, and CBD dilation of 7.7mm with sludge visualized within the CBD but no stones visualized. Endoscopic retrograde cholangiopancreatography was unremarkable. Liver biopsies were taken during subsequent endoscopic ultrasound. Pathology was consistent with ICI hepatitis. Pembrolizumab therapy was discontinued and she was started on pulse dose steroids and transitioned to oral steroids with marked improvement in her liver enzymes upon discharge. One week later, she returned to the emergency department with similar presenting symptoms and encephalopathy. Laboratory studies were significant for AST >5000, ALT 9203, Alkaline phosphatase 443, Total bilirubin 7.4, INR 2.2. She was started on steroids and N-acetylcysteine for non-acetaminophen-induced acute liver failure. Despite these therapies, the patient clinically deteriorated with worsening liver enzymes from acute liver failure.
Discussion: This case highlights the importance of recognizing ICI as a potential cause of severe hepatotoxicity and acute liver failure. Pembrolizumab, although historically associated with a lower incidence of ICI hepatitis, can lead to severe hepatotoxicity. In addition to stopping pembrolizumab, treatment with IV steroids and avoidance of other medications with potential liver toxicity is important to avoid worsening liver failure but liver transplant evaluation is of utmost importance to improve survival. Delayed presentation of fulminant liver failure can be long-term sequelae of immune checkpoint inhibitor use.

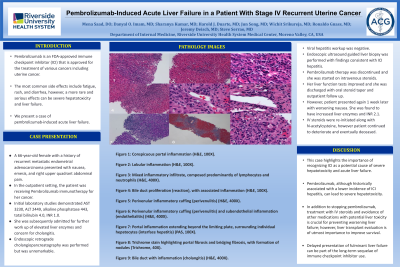
Figure: Figure 1: Conspicuous portal inflammation (H&E, 100X).
Figure 2: Lobular inflammation (H&E, 100X).
Figure 3: Mixed inflammatory infiltrate, composed predominantly of lymphocytes and neutrophils (H&E, 400X).
Figure 4: Bile duct proliferation (reaction), with associated inflammation (H&E, 100X).
Figure 5: Perivenular inflammatory cuffing (perivenulitis) (H&E, 400X).
Figure 6: Perivenular inflammatory cuffing (perivenulitis) and subendothelial inflammation (endothelialitis) (H&E, 400X).
Figure 7: Portal inflammation extending beyond the limiting plate, surrounding individual hepatocytes (interface hepatitis) (PAS, 100X).
Figure 8: Trichrome stain highlighting portal fibrosis and bridging fibrosis, with formation of nodules (Trichrome, 40X).
Figure 9: Bile duct with inflammation (cholangitis) (H&E, 400X).
Figure 2: Lobular inflammation (H&E, 100X).
Figure 3: Mixed inflammatory infiltrate, composed predominantly of lymphocytes and neutrophils (H&E, 400X).
Figure 4: Bile duct proliferation (reaction), with associated inflammation (H&E, 100X).
Figure 5: Perivenular inflammatory cuffing (perivenulitis) (H&E, 400X).
Figure 6: Perivenular inflammatory cuffing (perivenulitis) and subendothelial inflammation (endothelialitis) (H&E, 400X).
Figure 7: Portal inflammation extending beyond the limiting plate, surrounding individual hepatocytes (interface hepatitis) (PAS, 100X).
Figure 8: Trichrome stain highlighting portal fibrosis and bridging fibrosis, with formation of nodules (Trichrome, 40X).
Figure 9: Bile duct with inflammation (cholangitis) (H&E, 400X).
Disclosures:
Mena Saad indicated no relevant financial relationships.
Danyal Imam indicated no relevant financial relationships.
Sharanya Kumar indicated no relevant financial relationships.
Harold Duarte indicated no relevant financial relationships.
Jun Song indicated no relevant financial relationships.
Wichit Srikureja indicated no relevant financial relationships.
Ronaldo Gnass indicated no relevant financial relationships.
Jeremy Deisch indicated no relevant financial relationships.
Steve Serrao: Provation Medical – Advisory Committee/Board Member.
Mena Saad, DO1, Danyal O. Imam, MBBS1, Sharanya Kumar, MD1, Harold J. Duarte, MD2, Jun Song, MD3, Wichit Srikureja, MD1, Ronaldo Gnass, MD1, Jeremy Deisch, MD1, Steve Serrao, MD1. P4805 - Pembrolizumab-Induced Acute Liver Failure in a Patient With Stage IV Recurrent Uterine Cancer, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
