Tuesday Poster Session
Category: IBD
P4259 - Analyzing Racial Disparities Among FDA-Approved Clinical Trials for Novel Biologic Therapies of Ulcerative Colitis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Nadim A. Qadir, DO
University of Florida College of Medicine
Windermere, FL
Presenting Author(s)
Nadim A. Qadir, DO1, Landen Shane Burstiner, DO, MSc2, Anvit D. Reddy, DO2, Lauren N.. Stemboroski, DO2
1University of Florida College of Medicine, Windermere, FL; 2University of Florida College of Medicine, Jacksonville, FL
Introduction: The idea that ulcerative colitis (UC) primarily affects white populations has been extensively debunked in recent years, as the disease is increasingly diagnosed among minority populations. The primary objective of this study is to highlight the racial disparities among the clinical trials of biologic therapies throughout the years that were approved by the FDA to treat UC.
Methods: A structured search was performed through PUBMED and New England Journal of Medicine databases to identify those clinical trials that were submitted for FDA approval of novel biologic therapies for ulcerative colitis. ClinicalTrials.gov was used to establish countries and clinical sites involved in each trial. Patient demographics in these trials were analyzed, with a primary emphasis on race of included subjects. Patients were categorized as "Minority" if they did not identify as Caucasian/White.
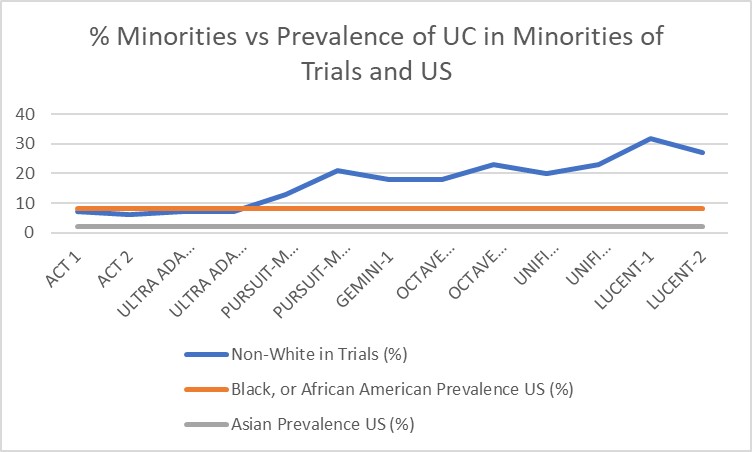
Results: From what we discovered as showcased in Table 1, compared to earlier trials for UC such as ACT and ULTRA, there is an uptrend of minority inclusion in recent studies corresponding to the increased number of clinical sites involved. As seen in Table 2, compared to the prevalence of minorities affected with UC in the United States, global trials for these novel biologics appear to have adequate representation of minorities. However, several studies did not describe the racial demographics in their publications with no further subgroup analysis other than stating white or non-white.
Discussion: Based on these results although it is promising that there is an uptrend of inclusion of minorities in these trials, but there is still a continued need for future clinical trials to focus on enrollment of not only African Americans, but other minorities as well. It is vital for these trials to continue to include minority demographics to allow for generalizability in this developing disease. According to the National Patient-Centered Clinical Research Network, UC has been estimated to affect 10% of minorities in the United States (US). However, between 1970 and 2010 there has been an increase in incidence rates of inflammatory bowel disease of 39% of whites and 134% non-whites in the US. If this trend is to continue, then it is imperative that the increased prevalence of minorities with UC be reflected in the studies that are being produced for these biologic therapies.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Nadim A. Qadir, DO1, Landen Shane Burstiner, DO, MSc2, Anvit D. Reddy, DO2, Lauren N.. Stemboroski, DO2. P4259 - Analyzing Racial Disparities Among FDA-Approved Clinical Trials for Novel Biologic Therapies of Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Florida College of Medicine, Windermere, FL; 2University of Florida College of Medicine, Jacksonville, FL
Introduction: The idea that ulcerative colitis (UC) primarily affects white populations has been extensively debunked in recent years, as the disease is increasingly diagnosed among minority populations. The primary objective of this study is to highlight the racial disparities among the clinical trials of biologic therapies throughout the years that were approved by the FDA to treat UC.
Methods: A structured search was performed through PUBMED and New England Journal of Medicine databases to identify those clinical trials that were submitted for FDA approval of novel biologic therapies for ulcerative colitis. ClinicalTrials.gov was used to establish countries and clinical sites involved in each trial. Patient demographics in these trials were analyzed, with a primary emphasis on race of included subjects. Patients were categorized as "Minority" if they did not identify as Caucasian/White.
Results: From what we discovered as showcased in Table 1, compared to earlier trials for UC such as ACT and ULTRA, there is an uptrend of minority inclusion in recent studies corresponding to the increased number of clinical sites involved. As seen in Table 2, compared to the prevalence of minorities affected with UC in the United States, global trials for these novel biologics appear to have adequate representation of minorities. However, several studies did not describe the racial demographics in their publications with no further subgroup analysis other than stating white or non-white.
Discussion: Based on these results although it is promising that there is an uptrend of inclusion of minorities in these trials, but there is still a continued need for future clinical trials to focus on enrollment of not only African Americans, but other minorities as well. It is vital for these trials to continue to include minority demographics to allow for generalizability in this developing disease. According to the National Patient-Centered Clinical Research Network, UC has been estimated to affect 10% of minorities in the United States (US). However, between 1970 and 2010 there has been an increase in incidence rates of inflammatory bowel disease of 39% of whites and 134% non-whites in the US. If this trend is to continue, then it is imperative that the increased prevalence of minorities with UC be reflected in the studies that are being produced for these biologic therapies.

Figure: Table 2. Trend of Minorities included in Biologic Trials with Association of Prevalence of Ulcerative Colitis in the United States
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Nadim Qadir indicated no relevant financial relationships.
Landen Shane Burstiner indicated no relevant financial relationships.
Anvit Reddy indicated no relevant financial relationships.
Lauren Stemboroski indicated no relevant financial relationships.
Nadim A. Qadir, DO1, Landen Shane Burstiner, DO, MSc2, Anvit D. Reddy, DO2, Lauren N.. Stemboroski, DO2. P4259 - Analyzing Racial Disparities Among FDA-Approved Clinical Trials for Novel Biologic Therapies of Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
