Back
Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 3A - Esophagus / Stomach / Practice Management
44 - Efficacy, Safety and Immunogenicity of Subcutaneous Infliximab (CT-P13 SC) Monotherapy versus Combination Therapy With Immunosuppressants Post hoc Analysis of LIBERTY-CD and LIBERTY-UC Studies (Late-Breaking Abstract)
Tuesday, October 29, 2024
3:25 PM – 3:35 PM ET
Location: Terrace Ballroom 1
.jpeg.jpg)
Bruce E. Sands, MD, MS, FACG
Chief, Division of Gastroenterology
Icahn School of Medicine at Mount Sinai
New York, NY
Late Breaking Abstract Presenter(s)
Bruce E. Sands, MD1, Jean-Frederic Colombel, MD1, Stephen B. Hanauer, MD, MACG2, William J. Sandborn, MD, FACG3, Stefan Schreiber, MD4, Silvio Danese, MD5, Sang Joon Lee, PhD6, Sung Hyun Kim, PhD6, YunJu Bae, MS6, Sun Hee Lee, MS6, Seul Gi Lee, MS6, Joon Ho Lee, MS6, Jong Min Kim, MS6, GaHee Park, MS6, Jimin Lee, MS6, Ju Hyun Lee, BA6, ChaeYoung Kim, BA6; 1Icahn School of Medicine at Mount Sinai, New York, NY, 2Northwestern University / Feinberg School of Medicine, Chicago, IL, 3University of California San Diego, San Diego, CA, 4University Hospital Schleswig-Holstein, Kiel, Germany, 5Vita-Salute San Raffaele University - IRCCS San Raffaele Scientific Institute, Milan, Italy, 6Celltrion, Inc, Incheon, South Korea
Introduction: Superior efficacy of subcutaneous (SC) infliximab (CT-P13 SC) over placebo for maintenance therapy in patients with Crohn’s disease (CD) and ulcerative colitis (UC) was demonstrated in the randomized LIBERTY-CD and LIBERTY-UC studies. The current post hoc analysis compared outcomes with CT-P13 SC by baseline immunosuppressant (IS) use.
Methods: Patients with moderately to severely active CD or UC randomized to the CT-P13 SC maintenance arm of the 54-week LIBERTY trials at Week (W) 10 and who were treated in the open-label extension (Weeks 56–102) were included. Endpoints were evaluated by baseline IS use (monotherapy versus combination therapy).
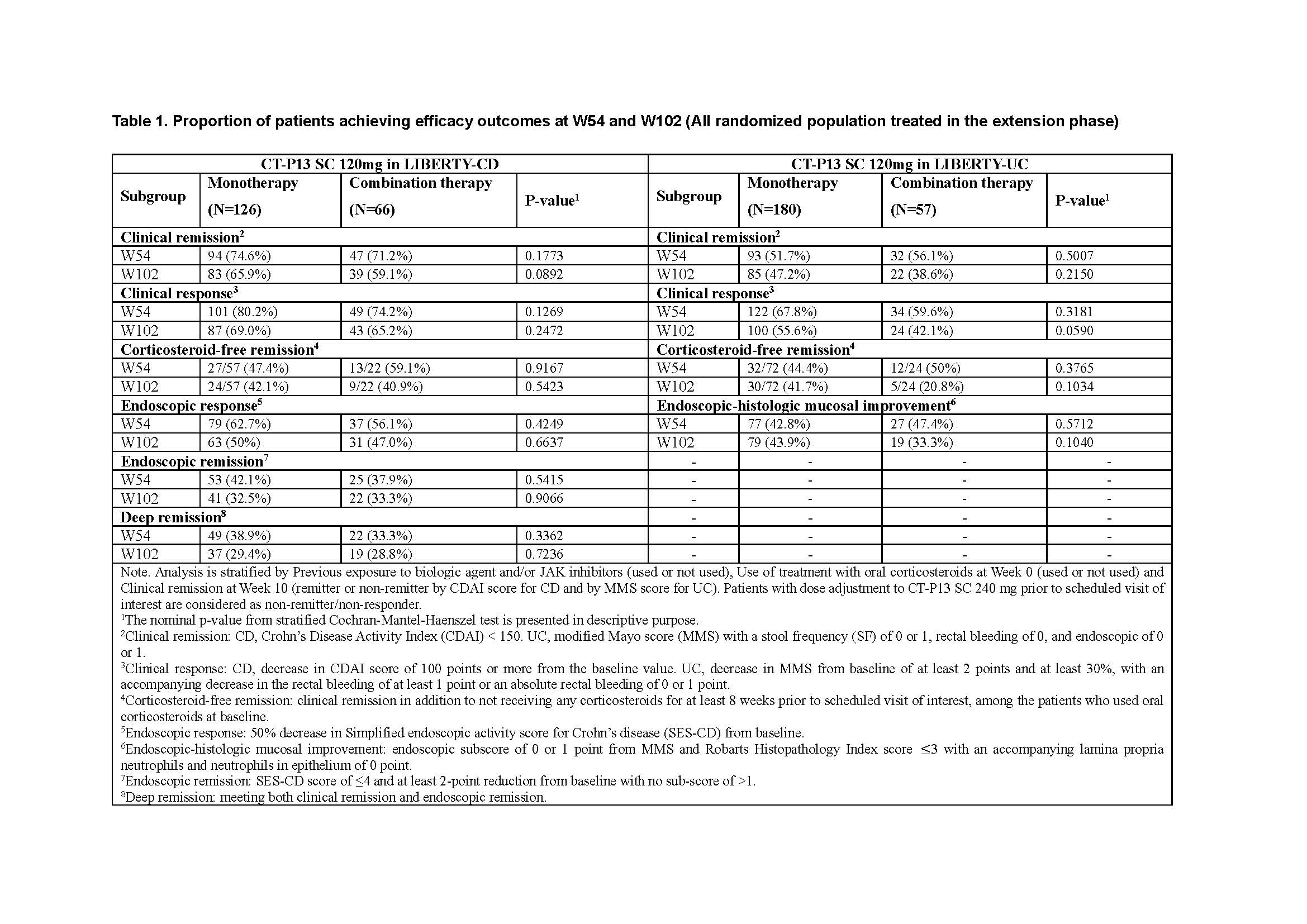
Results: A total of 192 patients with CD (monotherapy, n=126; combination therapy, n=66) and 237 patients with UC (monotherapy, n=180; combination therapy, n=57) were included. In both studies, there were no meaningful differences in efficacy outcomes between monotherapy and combination therapy at W54 or W102 (Table 1), despite combination therapy generally had higher mean trough level of infliximab from W10 to W102 compared to monotherapy (In CD, ranging from 12.1 to 14.8 μg/mL vs 12.4 to 16.3 μg/mL; In UC, ranging from 13.2 to 15.9 μg/mL vs 15.2 to 19.3 μg/mL). In combined analyses of CD and UC, no meaningful differences were observed in safety profiles between monotherapy and combination therapy including treatment-emergent adverse events (80.8% [253/313] vs 79.5% [101/127]), systemic injection reactions (3.8% [12/313] vs 1.6% [2/127]), and infection (42.5% [133/313] vs 47.2% [60/127]) in pooled maintenance and extension phases (W10 to W102). In combined analyses of CD and UC, rate of anti-drug antibody (ADA) positive conversion rates were higher with monotherapy relative to combination therapy up to W54 (71.7% [218/304] vs 49.6% [61/123]) and up to W102 (79.6% [242/304] vs 63.4% [78/123]).
Conclusion: No meaningful differences in efficacy outcomes were observed for patients during maintenance who had a clinical response to infliximab induction either as monotherapy or in combination with IS at W54 and W102. Combination with IS resulted in lower formation of ADA with higher drug levels that did not translate to different efficacy outcomes. The overall safety profile was also comparable between monotherapy and combination therapy.
Disclosures: B.E. Sands: Consultant or received speaker’s fees from AbbVie, Abivax, Adiso Therapeutics, AgomAb, Alimentiv, Amgen, AstraZeneca, Biora Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Cytoki Pharma, Eli Lilly and Company, Enthera, Evommune, Ferring, Galapagos, Gilead Sciences, Genentech, Glaxo SmithKline, Gossamer Bio, Imhotex, InDex Pharmaceuticals, Innovation Pharmaceuticals, Janssen, Johnson & Johnson, Kaleido, Kalyope, Merck & Co., Microba, Microbiotica, Morphic Therapeutic, MRM Health, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Sanofi, Surrozen, Synlogic Operating Company, Takeda, Target RWE, Theravance Biopharma R&D, TLL Pharmaceutical, USWM Enterprises, Ventyx Biosciences, Viela Bio; stock and stock options from Ventyx Biosciences. J.F. Colombel: Receiving payment for lectures from AbbVie, Amgen, Allergan, Inc. Ferring Pharmaceuticals, Shire, and Takeda; Receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, BMS, Celgene Corporation, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, Genentech, Glaxo Smith Kline, Janssen Pharmaceuticals, Kaleido Biosciences, Imedex, Immunic, Iterative Scopes, Merck, Microbia, Novartis, PBM Capital, Pfizer, Protagonist Therapeutics, Sanofi, Takeda, TiGenix, Vifor; Hold stock options in Intestinal Biotech Development. S.B. Hanauer: Consultancy from AbbVie, Allergan, Amgen, Arena, Astra Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cosmos, Catalys Pacific, Covance, Genentech, GSK, Janssen, Lilly, Merck, Novartis, Pfizer, Progenity, Prometheus, Receptos, Salix, Samsung Bioepis, Seres Therapeutics, Sorriso, Takeda, TLL, UCB, Vhsquared; Clinical Research for AbbVie, Allergan, Amgen, Celgene, Genentech, GSK, Janssen, Lilly, Novartis, Pfizer, Prometheus, Receptos, Takeda, UCB; Speaker for AbbVie, Bristol Myers Squibb, Janssen, Pfizer, Takeda; Independent Data Monitoring Conference for Arena, Boehringer Ingelheim, Bristol Myers Squibb, Gossamer, Prometheus, Protagonist. W. Sandborn: Consulting fees from Alimentiv, Shoreline Biosciences; Stock or stock options from Prometheus Biosciences, Prometheus Laboratories, Ventyx Biosciences, Mirador Therapeutics; Employee at Ventyx Biosciences, Mirador Therapeutics; Board of Directors at Prometheus Laboratories. S. Schreiber: Consultancy and personal fees from AbbVie, Arena, BMS, Biogen, Celltrion, Celgene, Falk, Ferring, Fresenius, Gilead, HIKMA, IMAB, Janssen, MSD, Morphic, Pfizer, Protagonist, Provention Bio, Sandoz, Takeda, and Theravance. S. Danese: Consultancy fees from AbbVie, Alimentiv, Allergan, Amgen, Applied Molecular Transport, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, Morphic, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, Teladoc Health, TiGenix, UCB Inc., Vial, Vifor. Reports lecture fees from Abbvie, Amgen, Ferring Pharmaceuticals Inc., Gilead, Janssen, Mylan, Pfizer, Takeda. S.J. Lee, S.H. Kim, Y.J. Bae, S.H. Lee, S.G. Lee, J.H. Lee, J.M. Kim, G.H. Park, J.M. Lee, J.H. Lee, C.Y. Kim are Employee of Celltrion, Inc.
Bruce E. Sands, MD, Jean-Frederic Colombel, MD, Stephen B. Hanauer, MD, MACG, William J. Sandborn, MD, FACG, Stefan Schreiber, MD, Silvio Danese, MD, Sang Joon Lee, PhD, Sung Hyun Kim, PhD, YunJu Bae, MS, Sun Hee Lee, MS, Seul Gi Lee, MS, Joon Ho Lee, MS, Jong Min Kim, MS, GaHee Park, MS, Jimin Lee, MS, Ju Hyun Lee, BA, ChaeYoung Kim, BA, 44, Efficacy, Safety and Immunogenicity of Subcutaneous Infliximab (CT-P13 SC) Monotherapy versus Combination Therapy with Immunosuppressants – A Post hoc Analysis of LIBERTY-CD AND LIBERTY-UC Studies (late-breaking abstract), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Introduction: Superior efficacy of subcutaneous (SC) infliximab (CT-P13 SC) over placebo for maintenance therapy in patients with Crohn’s disease (CD) and ulcerative colitis (UC) was demonstrated in the randomized LIBERTY-CD and LIBERTY-UC studies. The current post hoc analysis compared outcomes with CT-P13 SC by baseline immunosuppressant (IS) use.
Methods: Patients with moderately to severely active CD or UC randomized to the CT-P13 SC maintenance arm of the 54-week LIBERTY trials at Week (W) 10 and who were treated in the open-label extension (Weeks 56–102) were included. Endpoints were evaluated by baseline IS use (monotherapy versus combination therapy).
Results: A total of 192 patients with CD (monotherapy, n=126; combination therapy, n=66) and 237 patients with UC (monotherapy, n=180; combination therapy, n=57) were included. In both studies, there were no meaningful differences in efficacy outcomes between monotherapy and combination therapy at W54 or W102 (Table 1), despite combination therapy generally had higher mean trough level of infliximab from W10 to W102 compared to monotherapy (In CD, ranging from 12.1 to 14.8 μg/mL vs 12.4 to 16.3 μg/mL; In UC, ranging from 13.2 to 15.9 μg/mL vs 15.2 to 19.3 μg/mL). In combined analyses of CD and UC, no meaningful differences were observed in safety profiles between monotherapy and combination therapy including treatment-emergent adverse events (80.8% [253/313] vs 79.5% [101/127]), systemic injection reactions (3.8% [12/313] vs 1.6% [2/127]), and infection (42.5% [133/313] vs 47.2% [60/127]) in pooled maintenance and extension phases (W10 to W102). In combined analyses of CD and UC, rate of anti-drug antibody (ADA) positive conversion rates were higher with monotherapy relative to combination therapy up to W54 (71.7% [218/304] vs 49.6% [61/123]) and up to W102 (79.6% [242/304] vs 63.4% [78/123]).
Conclusion: No meaningful differences in efficacy outcomes were observed for patients during maintenance who had a clinical response to infliximab induction either as monotherapy or in combination with IS at W54 and W102. Combination with IS resulted in lower formation of ADA with higher drug levels that did not translate to different efficacy outcomes. The overall safety profile was also comparable between monotherapy and combination therapy.

Disclosures: B.E. Sands: Consultant or received speaker’s fees from AbbVie, Abivax, Adiso Therapeutics, AgomAb, Alimentiv, Amgen, AstraZeneca, Biora Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Cytoki Pharma, Eli Lilly and Company, Enthera, Evommune, Ferring, Galapagos, Gilead Sciences, Genentech, Glaxo SmithKline, Gossamer Bio, Imhotex, InDex Pharmaceuticals, Innovation Pharmaceuticals, Janssen, Johnson & Johnson, Kaleido, Kalyope, Merck & Co., Microba, Microbiotica, Morphic Therapeutic, MRM Health, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Sanofi, Surrozen, Synlogic Operating Company, Takeda, Target RWE, Theravance Biopharma R&D, TLL Pharmaceutical, USWM Enterprises, Ventyx Biosciences, Viela Bio; stock and stock options from Ventyx Biosciences. J.F. Colombel: Receiving payment for lectures from AbbVie, Amgen, Allergan, Inc. Ferring Pharmaceuticals, Shire, and Takeda; Receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, BMS, Celgene Corporation, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, Genentech, Glaxo Smith Kline, Janssen Pharmaceuticals, Kaleido Biosciences, Imedex, Immunic, Iterative Scopes, Merck, Microbia, Novartis, PBM Capital, Pfizer, Protagonist Therapeutics, Sanofi, Takeda, TiGenix, Vifor; Hold stock options in Intestinal Biotech Development. S.B. Hanauer: Consultancy from AbbVie, Allergan, Amgen, Arena, Astra Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cosmos, Catalys Pacific, Covance, Genentech, GSK, Janssen, Lilly, Merck, Novartis, Pfizer, Progenity, Prometheus, Receptos, Salix, Samsung Bioepis, Seres Therapeutics, Sorriso, Takeda, TLL, UCB, Vhsquared; Clinical Research for AbbVie, Allergan, Amgen, Celgene, Genentech, GSK, Janssen, Lilly, Novartis, Pfizer, Prometheus, Receptos, Takeda, UCB; Speaker for AbbVie, Bristol Myers Squibb, Janssen, Pfizer, Takeda; Independent Data Monitoring Conference for Arena, Boehringer Ingelheim, Bristol Myers Squibb, Gossamer, Prometheus, Protagonist. W. Sandborn: Consulting fees from Alimentiv, Shoreline Biosciences; Stock or stock options from Prometheus Biosciences, Prometheus Laboratories, Ventyx Biosciences, Mirador Therapeutics; Employee at Ventyx Biosciences, Mirador Therapeutics; Board of Directors at Prometheus Laboratories. S. Schreiber: Consultancy and personal fees from AbbVie, Arena, BMS, Biogen, Celltrion, Celgene, Falk, Ferring, Fresenius, Gilead, HIKMA, IMAB, Janssen, MSD, Morphic, Pfizer, Protagonist, Provention Bio, Sandoz, Takeda, and Theravance. S. Danese: Consultancy fees from AbbVie, Alimentiv, Allergan, Amgen, Applied Molecular Transport, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, Morphic, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, Teladoc Health, TiGenix, UCB Inc., Vial, Vifor. Reports lecture fees from Abbvie, Amgen, Ferring Pharmaceuticals Inc., Gilead, Janssen, Mylan, Pfizer, Takeda. S.J. Lee, S.H. Kim, Y.J. Bae, S.H. Lee, S.G. Lee, J.H. Lee, J.M. Kim, G.H. Park, J.M. Lee, J.H. Lee, C.Y. Kim are Employee of Celltrion, Inc.
Bruce E. Sands, MD, Jean-Frederic Colombel, MD, Stephen B. Hanauer, MD, MACG, William J. Sandborn, MD, FACG, Stefan Schreiber, MD, Silvio Danese, MD, Sang Joon Lee, PhD, Sung Hyun Kim, PhD, YunJu Bae, MS, Sun Hee Lee, MS, Seul Gi Lee, MS, Joon Ho Lee, MS, Jong Min Kim, MS, GaHee Park, MS, Jimin Lee, MS, Ju Hyun Lee, BA, ChaeYoung Kim, BA, 44, Efficacy, Safety and Immunogenicity of Subcutaneous Infliximab (CT-P13 SC) Monotherapy versus Combination Therapy with Immunosuppressants – A Post hoc Analysis of LIBERTY-CD AND LIBERTY-UC Studies (late-breaking abstract), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

